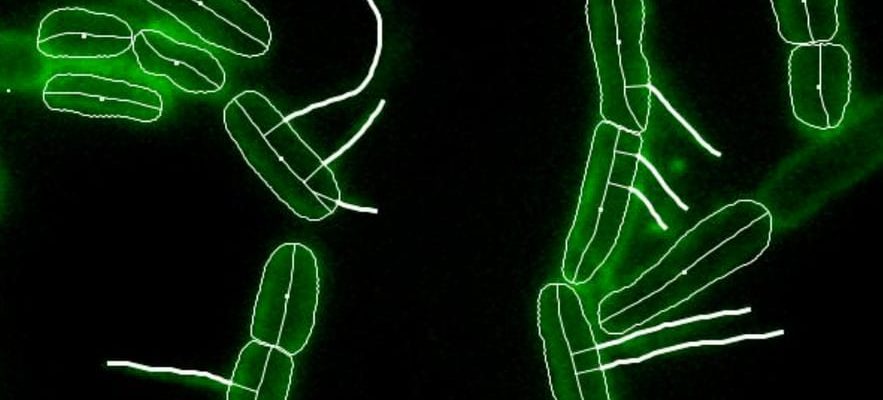
The equivalent of a night of sex, revealed to the whole world, without makeup. For the first time, a “remote conjugation” was filmed between two bacteria, a kind of mating among microorganisms, captured live by Inserm scientists. If of course, there are no acts of copulation in these living beings, they can do without genes when they come into contact, or through a rod called “pilus”, a fiber which can grow at the appropriate time and penetrate its congeners.
Published Tuesday November 14, 2023 in the journal Proceedings of the National Academy of Sciences (PNAS), this work provides the first direct and unequivocal proof of this mechanism, at the heart of one of the greatest contemporary health challenges. Thanks to this genetic exchange, bacteria can share the mutations that make them resistant to antibiotics, without waiting to multiply. A process which, if we cannot thwart it, could ultimately contribute to making antibiotic treatments ineffective.
Using these filaments, filmed by researchers from Lyon 1 for the first time on November 14, 2023, E.choli bacteria exchange genes.
© / PNAS/Inserm screenshot
Obtained thanks to the fluorescent marking of different bacteria and the recent appearance of more efficient microscopes, these results could advance the fight against antibiotic resistance, one of the “greatest threats to humanity”, according to the World Organization for Disease Control and Prevention. health (WHO). “To develop new therapeutic methods, we must first know how antibiotic resistance works,” argues Christian Lesterlin, Inserm research director and first author of the study.
Passing on bad genes
When antibiotics are ingested, they do not kill all pathogenic bacteria, especially if the treatment is stopped prematurely. Some may prove insensitive, due to a different genetic heritage, giving them the ability to resist the toxicity of treatments. They multiply, spread from one host to another, and become more and more common. Thus, 1.2 million people die each year from resistant infections according to a study published in 2019 in The Lancet. A figure that could increase tenfold by 2050, according to the WHO.
In parallel with his fundamental research, the researcher and his molecular microbiology and structural biochemistry unit (CNRS/Lyon 1) are attempting to modify bacteria so that they transmit, through conjugation, poor genetic information. This way, the targeted pathogens would die or become susceptible to antibiotics again. An innovative avenue, financed by the Joint Programming Initiative on Antimicrobial Resistance (JPIAMR), one of the main international initiatives, supported by 29 countries.
Until now focused on the discovery of new antibiotics, more and more research programs are exploring alternative avenues. Christian Lesterlin’s team is one of them. She has started experiments on mice and hopes to be able to launch clinical trials in a few years. Although this strategy has proven itself in vitro, scientists do not yet know how the solutions developed will behave in the body. They could prove toxic or not reach the infected organs in large enough quantities.
More and more alternative research
At the CNRS Institute of Chemistry of Natural Substances, in Gif-sur-Yvette (Paris region), Véronique Eparvier tries to draw inspiration from nature to discover new methods for eliminating microbes. “Despite the emergence of artificial intelligence, we are not yet able to simulate the full diversity of nature. However, many bacteria naturally produce toxins against other microorganisms,” explains the research director. Although his work is essentially fundamental, hospitals regularly send him resistant strains, so as not to miss out on a potential cure.
In parallel with these alternative avenues, new antibiotic molecules, a French and global scientific priority, have become increasingly rare since their golden age in the 1980s. Only around fifteen have been discovered over the last twenty years. The most recent, Teixobactin and Clovibactin have yet to pass the human testing phases. But like them, most mainly attack Gram-positive bacteria, a category which does not yet pose too much of a problem – most of the resistance occurs against “Gram-negative” bacteria.
A slowdown attributable to the speed of adaptation of bacteria, but not only that: “Conducting trials can be complicated. Few people agree to test one molecule when another is effective,” adds Philippe Glaser, coordinator of the strategy against antibiotic resistance at the Pasteur Institute, focused on the search for molecules capable of altering the ability of bacteria to use our cells. Most of the time, new antibiotics are reserved for hospital use as a last resort, to prevent bacteria from adapting – this is what they do almost systematically.
Sobriety, the most effective response
Researchers are also trying to identify antibiotics that generate less resistance, by attacking new targets in bacteriological cells: “Instead of killing the bacteria and leaving only the protected ones, we are trying to handicap them. Thus , they will remain numerous and will prevent the emergence of insensitive ones, while being less dangerous for us”, indicates Olivier Neyrolles, research director at the Institute of Pharmacology and Structural Biology of Toulouse, specialist in tuberculosis, one of the diseases among the most resistant, because it is responsible for long infections, which are more conducive to mutations.
Other work focuses on the rehabilitation of phage therapies, these treatments based on the use of viruses called “phages” that devour… bacteria. Known since the 1920s, this solution has been abandoned by the industry, in particular because it requires knowing the exact type of microorganism causing the infection, which extends treatment times. . If these elements open up the field of possibilities, ultimately they will also be exposed to bacterial resistance mechanisms, even more rapid in the case of phages.
In the opinion of all researchers, surveillance, prevention and sobriety still remain the most effective means of combating antibiotic resistance. Around half of antibiotic prescriptions are unnecessary or inappropriate, Public Health France recalled this Wednesday in an inventory. If prevention campaigns – “Antibiotics are not automatic” – had reduced consumption in cities in recent years, it rose by 16.6% in 2022. France, a historic pharmaceutical power, remains the fifth nation the largest consumer of antibiotics in Europe. The only good news: between 2012 and 2022, the administration of antibiotics to animals fell by 48.6%, according to the latest figures from Public Health France.
.