Published on
Updated
Reading 2 mins.
In the case between patient associations and the Merck laboratory, concerning the change in the formula of the drug Levothyrox, the National Medicines Safety Agency has just been indicted by an examining magistrate, Monday, December 5, for “deception”. Interviewed, Maître Christophe Lèguevaques, lawyer for victims, gives us his point of view.
The ANSM has just been indicted for “deception“this Monday, December 5, following the decision of an investigating judge in the context of the Levothyrox drug case and more specifically its change of formula. She therefore joined the French subsidiary of the German pharmaceutical laboratory Merck, also placed in exam for “aggravated deception” in the same folder.
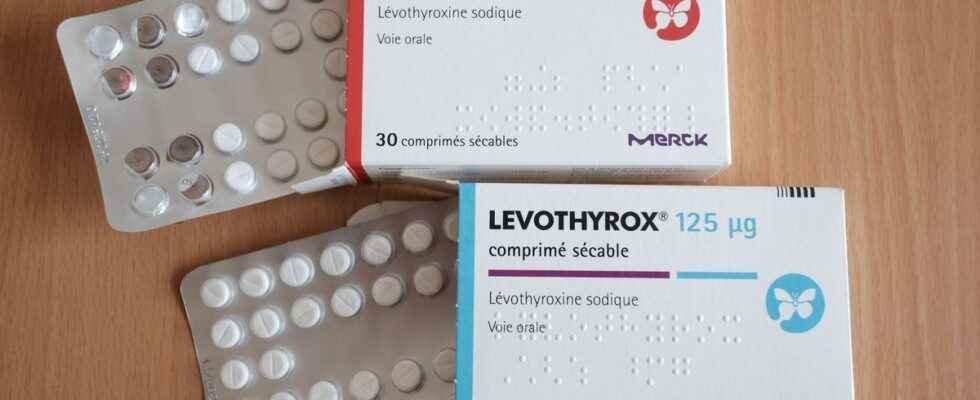
The heart of the file: the composition of Levothyrox
The story begins in 2017 with the arrival in France at the end of March of a new composition of the drug Levothyrox. The active ingredient of the drug, levothyroxine, remains the same, but other excipients are present in the drug.
Many patients then complain of side effects, such as dizziness, headaches, digestive disorders or hair loss. In September 2017, no less than 9,000 people filed complaints.
A questioning of legal persons
Following this observation, a criminal investigation will be opened in March 2018, in order to shed light on this case. In June 2019, the ANSM conducted a study on more than two million patients and concluded that the switch to the new Levothyrox formula did not cause “serious health problems” and boast “the good quality of the new formula“. The Merck laboratory also denies any anomaly of its product.
For master Christophe Lèguevaques, lawyer for the victims of Levothyrox, this indictment of the ANSM is not a surprise: “Justice is taking its course and the ANSM, like Merck, will have to be held accountable. The agency took at face value what the laboratory was telling it, in particular their bioequivalence studies, while the patients were almost considered fabricators“. The lawyer would however like to point out that the ANSM is presumed innocent at this stage of the investigation and that natural persons will probably be involved in the consequences of the case.
Publication of a press release of a few lines
Following this indictment, the ANSM published a statement of a few lines in which it recalls that it has “never denied the difficulties encountered by some patients when switching to the new formula of Levothyrox and is constantly and daily concerned about the safety and health of patients“. She also states that she “will make its full contribution to the manifestation of the truth but strongly contests the reproaches made against it, because no criminal offense has been committed”.
An old formula of Levothyrox, still available
Used daily by 2.5 million patients in France, the drug Levothyrox (and more particularly its new formula) was mostly well accepted by patients. Today, less than 100,000 patients are treated with the old formula, called Euthyrox. “Since 2018, boxes of the old formula have been available for patients who wish to continue taking it” underlines Maître Lèguevaques.
“Initially it was presented by Merck as a favor for the sick, and eventually they were able to have it every year and it will still be the case next year. This is what we do not understand: why not continue to produce the old formula in parallel with the new? wonders the lawyer, who has already condemned the German laboratory, at the beginning of the year, and compensated up to 1000 euros each of the victims of the civil part of the case.