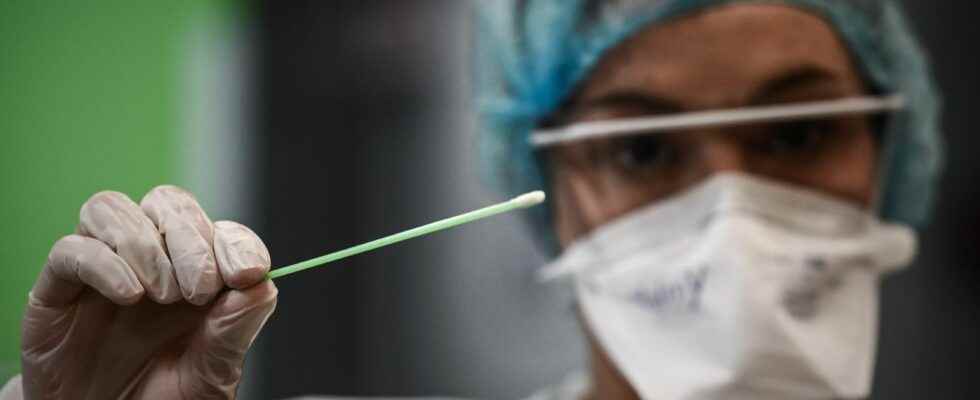
For several days, new antigenic tests have been making a lot of noise. These devices, equipped with a single swab, make it possible to detect in a single sample two viruses with very similar symptoms: influenza or Covid-19. The sample is taken in the same way as for the Covid-19 tests: nasal mucus is collected with the swab, mixed then deposited on a strip which will detect the antigens of one or the other. 10 to 15 minutes later, the diagnosis is made and patients can find out which of these two viruses they have contracted.
The two main tests, manufactured by the Toda Pharma and AAZ laboratories, are already available in pharmacies. They cost around 5 euros and are not fully reimbursed by social security, since the antigenic tests detecting the flu are not covered by health insurance. “The High Authority for Health (HAS) must issue an opinion at the end of January in which it will indicate whether these tests will be reimbursed and it must also decide whether or not to authorize the new triple tests that we are preparing, which will make it possible to detect bronchiolitis. , in addition to the flu and the Covid”, indicates to L’Express Fabien Larue, director of the AAZ group.
What advantages?
In theory, these tests can allow patients and healthcare professionals to have a sure diagnosis – in the event of a positive flu or Covid-19 result – or by elimination – in the event of a negative result. They could therefore make it possible to adapt the treatment or the recommendations, for example by excluding the use of antibiotics – commonly prescribed during bacterial infections, but useless in the fight against viruses, except in the event of bacterial superinfection -, to reassure a patient potentially at risk, or even simply to avoid a medical consultation and thus relieve city and emergency doctors.
What efficiency?
In fact, these benefits are possible if the tests are reliable. The laboratories, themselves, ensure that this is the case. Both Toda Pharma and AAZ have 100% specificity, which is the certainty that their tests will not produce false negatives. As for sensitivity, that is to say the ability to detect the virus if the person is infected, Toda Pharma claims to obtain a rate of 98.9% for Covid-19, 99.9% for influenza A and 99% for influenza B.
Asked by L’Express, his competitor Fabien Larue, director of the competing group AAZ, which indicates that he obtains around 90% sensitivity for the flu and the Covid, is skeptical. “Historically, most flu tests have an efficiency of 60 or 70%. Getting 90% is already an exceptional performance. Plus, it seems unrealistic to me,” he points out, adding that the results tests to detect bronchiolitis – which have yet to be validated by health authorities – have a specificity of 100% and a sensitivity of 80%.
Also interviewed by L’Express, Maxime Sol, biologist in a private laboratory of the INOVIE group, is even more skeptical. “Historically, for influenza, the performance of antigenic tests that take nasopharyngeal samples is limited, between 50 and 80% depending on the strains circulating, the kits and the person taking the sample, he explains. As for Covid- 19, I really dislike the attitude of the laboratories. They give results close to 100% based on the results of scientific studies, such as the study by Henri Mondor Hospital (AP-HP, Inserm), published in the journal Microbiology Spectrum, August 31, 2022. Except that they only reach 100% on one out of three evaluation series. In addition, they retain only the most virus-rich samples! Including all patients, the study finds an overall sensitivity of around 65%.
Akli Bouaziz, scientific director of AAZ, recalls for his part that the study in question underlines that the AAZ tests are the most reliable on the market – they are, in fact, used as reference tests by the authors of the study – and adds and that they detect Omicron and its subvariants very well. “Scientific studies can present biases and moreover, the authors of the Henri Mondor study specify that the swab samples were not only frozen, which partially destroys the virus, but were diluted in cultures, which makes also lower the viral load of the sample. All this reduces the detection rate compared to a sample in real conditions, he defends himself. Our tests are close to 100% detection for high and medium viral loads, and lower for light and very light loads.” The specialist nevertheless recognizes that these results drop slightly for nasal tests – less invasive and more intended for children – compared to nasopharyngeal tests.
The debates around the effectiveness of antigens for influenza or Covid-19, less than PCR, are not new. One of the difficulties stems from the fact that there are a large number of manufacturers. In this regard, one certainty is emerging: those made in China, common in our pharmacies, appear to be of lower quality than the European tests. Despite these legitimate questions, the interest of being tested in case of doubt, in particular for Covid-19, remains obvious, by allowing you to isolate yourself as well as possible and to apply barrier gestures with more vigilance.