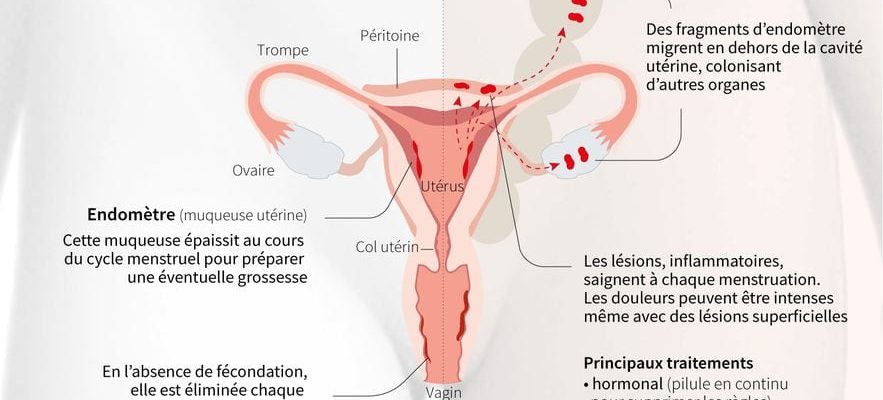
In a low range, 1 in 10 women of childbearing age would be affected byendometriosis In France. This chronic gynecological pathology which, according to definition from the Ministry of Health, is characterized by “the development of the internal uterine lining (the endometrium) outside the uterus, sometimes colonizing other organs”. It can cause painful periods, disabling pelvic, abdominal and/or lumbar cramps, but also pain during sexual intercourse and, in the most serious cases, infertility, or even digestive and urinary problems.
“In reality, it would be more than 1 in 7 women, or even 1 in 5 women,” reports Erick Petit, radiologist specializing in endometriosis and member of the steering committee for the national strategy against this pathology. The problem is that we does not know the epidemiology of endometriosis well as the lack of knowledge of the pathology leads to diagnostic errors for up to ten years.”
Endometriosis
© / afp.com/Paz PIZARRO, Anibal MAIZ CACERES, Emmanuelle MICHEL
Reduce diagnostic wandering
It is precisely to reduce this diagnostic wandering experienced by many women with endometriosis that the High Authority for Health (HAS) has opened the door to the management of a saliva test deemed “promising”. Developed by the Lyon biotech Ziwig, this test, called Endotest, “showed very good diagnostic performance”, underlines the HAS, which took action to evaluate its effectiveness and usefulness. The test is therefore based on a saliva sample, which contains microRNAs. Thanks to saliva sampling, it is possible “to get as close as possible to the biological functioning of cells and to produce information that is not obtained either through imaging or through surgery and which allows a reliable biological diagnosis to be made” , explained to AFP Yahya El Mir, the founder of Ziwig.
In fact, this test would not be aimed at all women, but only at those in whom clinical examinations or medical imaging (i.e. ultrasound and/or pelvic MRI) do not cannot explain the persistent pain. The objective would thus be to use the Endotest before the laparoscopy stage, an invasive and risky examination, not to mention that it may prove useless when there are no traces of lesions characteristic of disease. “Reducing the diagnosis time to around ten days, while on average women with endometriosis wander for seven years, is clearly a revolution,” assures Dr. Erick Petit, from the steering committee of the research project on endometriosis. “Endotest. Especially since detecting the pathology earlier would speed up the care process,” he adds.
No refund provided
The HAS issued its opinion on Monday January 8, based on clinical results demonstrating a diagnostic accuracy of 95%, on nearly 1,000 patients. An opinion which was eagerly awaited in France since the publication of the results of a study on the saliva test developed by Ziwig, in June 2023, in the scientific journal The New England Journal of Medicine.
Today, if the HAS unequivocally recognizes the “innovative character” and the “diagnostic effectiveness” of this test, it nevertheless emphasizes “the need to carry out additional studies aimed at evaluating its clinical usefulness in current practice”, before to systematize it and, above all, before it is reimbursed by Social Security.
However, it is the marketing and reimbursement of the test that could “change the situation” for patients, annoys Priscilla Saracco, general director of the Endomind association and patient expert in endometriosis. “There is a form of incomprehension,” she adds. “Why couldn’t we quickly take all the necessary measures to make this test widely accessible?”
But access via an innovation package
Some people point to the high cost of the test and the uncompromising rigor of the French HAS. “For the test to be reimbursed, you must go to the HAS, it is obligatory, and it is among the most rigorous institutions in the world,” underlines Erick Petit. Nevertheless, the doctor recalls that this test is an incredible opportunity to advance patient care. Because diagnostic error is also very expensive. “The longer the diagnosis, the greater the risk of chronic pain, not to mention the risk to fertility. Some women live with very debilitating endometriosis which prevents them from working and living properly on a daily basis,” he explains.
Judging “the data too preliminary to grant a favorable opinion for reimbursement under common law”, the HAS nevertheless wishes to set up early and secure access for women to this test, as part of an “innovation package”, which will in turn allow in addition to collecting missing data. The innovation package aims to facilitate access to innovative technologies with financial support. “Thus, patients could benefit from early, supervised and secure access to Endotest, with exceptional care conditional on the completion of the clinical utility study,” specifies the HAS on its site. This would then make it possible to bring together the so-called “missing” data, namely the impact of the test on patient care, an estimate of the volume of test prescriptions in the target population, as well as the acceptability of the test by patients. “Obtaining them will make it possible, within a reasonable time, to decide on the long-term reimbursement of Endotest,” writes the health authority.
If France remains behind for the moment, the Endotest is already sold in around ten European countries, including Switzerland, Sweden, the United Kingdom, Norway, and even Italy , and from the Middle East, “around 1,000 euros” per unit, according to the biotech Ziwig.
.