Tag: EMA
EMA, Pfizer bivalent vaccine evaluation begins
(Finance) – They are 31,088 i new Coronavirus positives in Italy in the last 24 hours compared to just over 208,000 swabs carried out, with the positivity rate at 14.9%.…
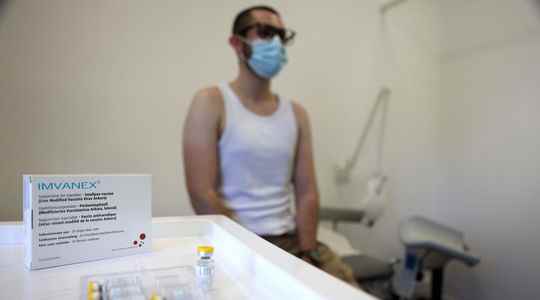
Monkey pox: what is the new injection technique authorized by the EMA?
A smaller sting for a bigger effect… and bigger irritations? The European Medicines Agency (EMA) authorized, on Friday August 19, a new technique for injecting the vaccine against monkeypox. It…
Covid, Cavalieri (Ema): a fourth dose remains valid for the elderly and vulnerable
(Finance) – They are 107,240 the new infections from Covid recorded in the last 24 hours according to data from the Ministry of Health. Yesterday 107,786 were infected. The victims…
EMA approves Valneva’s vaccine – DN.SE
The EMA recommends that the European Commission go ahead and approve Valneva’s vaccine for use within the Union. The vaccine is approved for people in the age range 18-50 years.…
EMA approves Valneva’s vaccine
The European Medicines Agency (EMA) has approved the French pharmaceutical company Valneva’s vaccine against covid-19, the agency writes in a press release. The EMA recommends that the European Commission go…
Valneva Covid vaccine: examined by the EMA, when in France?
The Franco-Austrian laboratory Valneva has developed the only inactivated virus vaccine against Covid-19. The EMA will begin to examine its application for marketing in Europe, announces the company. Name, date…
the anti-Covid pill fully authorized by the EMA
ANTI-COVID PILL. Authorized for people at risk of serious forms by the High Authority for Health, the extended use of the curative treatment is approved by the European Medicines Agency…
Covid, EMA green light for Pfizer’s pill
(Finance) – The European Medicines Agency (EMA) has given conditional authorization toplacing Paxlovid on the market, the oral drug manufactured by Pfizer for the Covid-19 cure. The medicine is recommended…
Novavax vaccine: validated by the EMA, when in France?
The Novavax vaccine (Nuvaxovid) received the green light from the European Medicines Agency (EMA) on December 20 to be placed on the market. 90% effective against the initial strain of…