You will also be interested
[EN VIDÉO] Stem cells could repair a damaged spinal cord The spinal cord is part of the central nervous system. When it is damaged, the connection between the body and the brain is altered. Until now, a lesion of this part of the body left little hope of recovery. Discover, in video and thanks to Discovery Science, how stem cells could enable remission.
Researchers from the University of Cambridge and Caltech have created embryos artificial mice that have a beating heart, the basics of a brain and all the other organs of the mouse body, and this, from stem cells (which can differentiate into any cell type). According to the team, their results reported in Nature are the fruit of more than a decade of research, which finally resulted in increasingly complex embryonic structures.
A self-organization of embryonic stem cells, without fertilization
In humans, three types of stem cells develop during the first week after the fertilization : one will become the tissues of the body and the other two will support the development of theembryo (extra-embryonic cells). For the experiment, the researchers only induced the three types of cells to self-assemble, without requiring anyovum nor of sperm. The embryos synthetics developed until day 8.5, endowed with a beating heart, the bases of a brain, as well as the yolk sac where the embryo develops and from which it receives nutrients during its first weeks.
This is the most advanced stage of development achieved to date in a stem cell-derived model. ” Our results demonstrate the capacity for self-organization of embryonic stem cells and two types of extra-embryonic stem cells to reconstruct the development of mammals through and beyond the gastrulationup to neurulation and theorganogenesis early write the authors of the study.
” Access to the developing structure »
If the progress is remarkable, what could it be used for? ” The stem cell embryo model is important because it gives us access to the developing structure at a stage that is normally hidden from us due to the implantation of the tiny embryo in theuterus from the mother “, Explain in a press release Magdalena Zernicka-Goetz, co-author of the study. ” This accessibility allows us to manipulate genes to understand their roles in development in a model experimental system. »
Many pregnancies fail when stem cells start sending these kinds of signals to each other
One of the major interests is to better understand why certain embryos develop in fetus, while others do not, and to be able to prevent it. Zernicka-Goetza’s team discovered that extraembryonic cells send chemical and mechanical signals to embryonic cells, which guide embryo development. However, many pregnancies fail when the stem cells start sending these kinds of signals to each other.
Finally, the method could be reused with human stem cells. These cells would then be used to guide the repair and development of synthetic human organs for patients awaiting transplantation. Worldwide, the need for graft of organs is growing; in France alone, more than 20,000 people are currently waiting for a transplant.
They created artificial mouse embryos!
Article of Marie-Celine Raypublished on 06/03/2017
Cambridge University researchers obtained an embryo-like structure from two types of stem cells growing on a 3D matrix. What better to understand the first stages of embryonic development.
After fertilization, the egg cell divides into stem cells embryonic. Can such cells in vitro, reconstitute an embryo? Previous work has attempted to reconstitute an embryo using only embryonic stem cells, but with little success: the embryo needs other types of cells to coordinate its development.
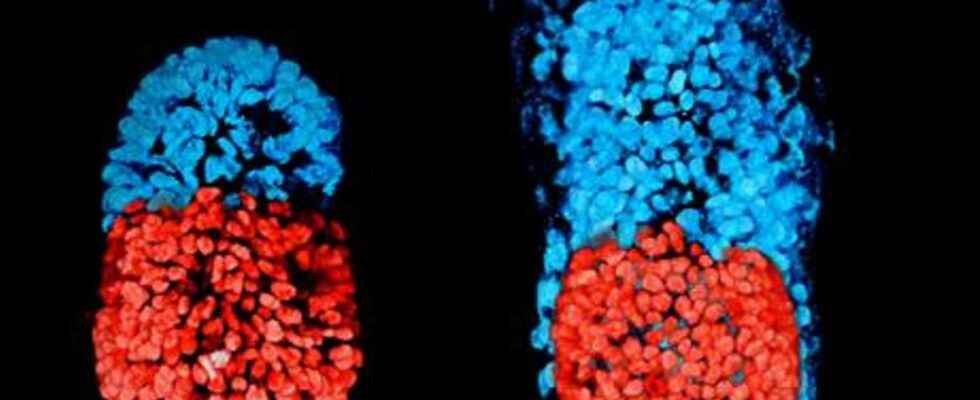
This is why a team of Cambridge created an artificial embryo using two types of mouse cells: embryonic stem cells and extra-embryonic stem cells from the trophoblast, at the origin of the placenta. All in the presence of a extracellular matrix. In this way, the structure comes together and its development resembles a natural embryo, with the embryonic stem cells at one end and trophoblast stem cells at the other. The researchers observed that the cells communicated with each other, which allowed them to position themselves correctly: “The embryonic and extra-embryonic cells begin to talk to each other and organize themselves into a structure that looks and behaves like an embryo,” explained Magdalena Zenricka-Goetz, who led this work. A proamniotic cavity, in which theembryo grows, forms.
The embryo should not give rise to a viable fetus
But this artificial embryo should not be able to develop into a normal fetus, as it would likely need a third type of stem cell to form the yolk sac, which is necessary for nutrition. The team also works on human embryos. In the United Kingdom, experiments on human embryos are regulated and prohibited beyond 14 days. But if this research continues, it could make it possible to obtain embryos beyond this 14-day limit.
These works published in Science help to better understand early embryonic development and its anomalies.
—
LAST DAYS to take advantage of our summer offer.
Subscribe to our media for a period of 3 months and receive the Mag Futura as a gift!*
*Offer valid for any new 3-month subscription to the “I participate in the life of Futura” offer on Patreon.
—
Interested in what you just read?