Baldness is a common problem for millions of people around the world. There has been a promising development regarding baldness. Doctor Mehmet Emin Adin, who lives in the USA and works as a physician at Yale University, announced the announcement on his Twitter account. Stating that successful progress has been made in the treatment, Dr. Adin stated that the FDA has approved the drug and it will be used soon.
Dr. Adin’s posts are as follows:
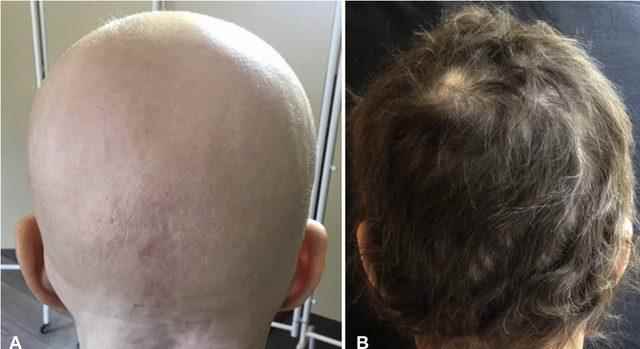
“Very good news for those suffering from baldness. This is the first time the FDA has approved a drug used in the treatment of baldness. The JAK inhibitor Baricitinib has been approved for the treatment of alopecia areata (regional baldness). Images of a sample patient before and after treatment.” He shared the photo.
The name of the drug is Olumiant. It can be used in people (no matter whether men or women) who have complete or almost complete hair loss (eyebrows, eyelashes, etc.). According to the study, at least 80% hair growth was observed in one third of the patients using 4 mg. The drug will cost $5 per month in the US, $25 per month for those without insurance.
“DATE IS WRITTEN”
“One of the people who led the study of the drug is Dr King from our university. “Shortly after I started treating a patient who lost his hair, beard and eyebrows, his hair started to grow and history was made, this drug changed the disease forever.”
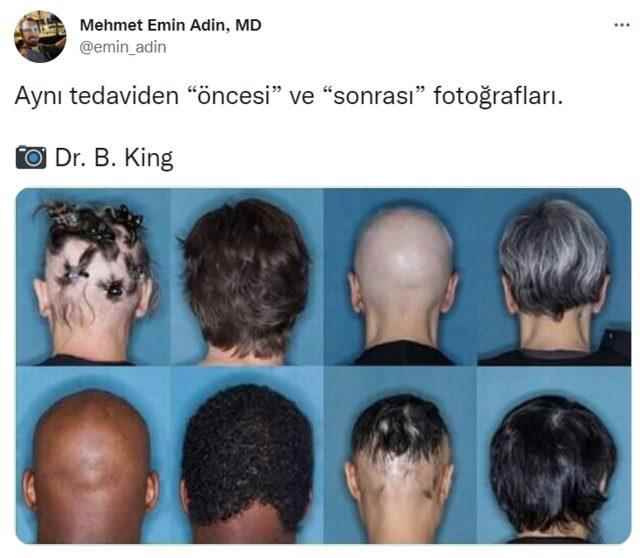
Dear friends, let me state that I have absolutely no ties to those who produce the drug. I was informed by the e-mail I received because of his connection with our university. Also, as I mentioned above, this treatment is not about androgenic type baldness.
The scientists who conducted two phase 3 studies conducted in more than one country (in the image I shared above) stated that those with androgenic type (male type) hair loss were not included in the study, but they may have been included in the study by mistake.”