Published on
updated on
Reading 2 min.
Apple is taking a new step forward in connected health with the approval by the American authorities of its new sleep apnea detection function for the Apple Watch. Why is the approval of this feature so important? A look back at an innovation that could well be a game-changer.
This decision by the Food and Drug Administration (FDA) marks a major step forward for millions of people suffering from this often underdiagnosed disorder.
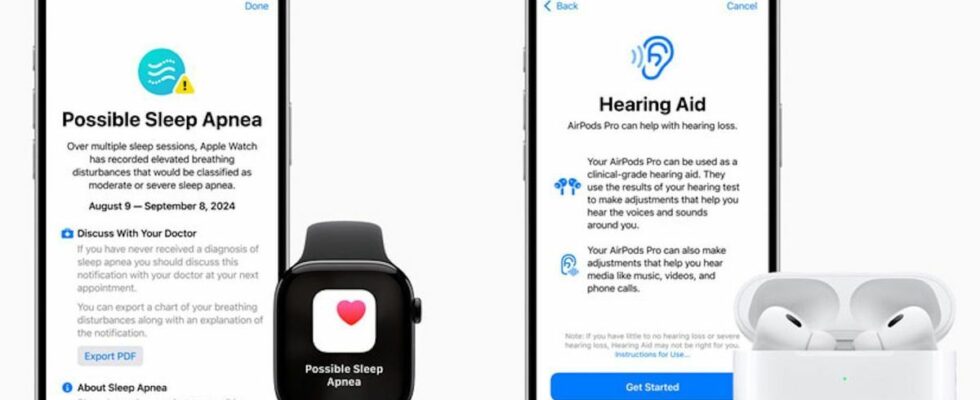
Sleep apnea detection integrated into the Apple Watch
Sleep apnea is a serious disorder that causes repeated pauses in breathing during sleep. If left untreated, it can lead to complications such as high blood pressure, heart problems, and even an increased risk of stroke. Yet many people are unaware that they have it because they don’t have clear symptoms during the day. That’s where Apple’s technology comes in.
Already known for its fall detection and heart rate monitoring features, the Apple Watch has just received FDA approval for its ability to detect sleep apnea. A crucial innovation in the field of public health, given that sleep apnea is far from rare.
According to Insermin adults, the incidence of sleep apnea syndrome increases almost linearly with age: 7.9% of people aged 20 to 44, 19.7% of people aged 45–64 and 30.5% of people over 65 are affected.
The FDA approval follows rigorous clinical testing by Apple, demonstrating that its watch can detect sleep apnea episodes using a series of advanced sensors. As detailed in the Apple press release“The Breathing Disturbances metric uses the Apple Watch accelerometer to detect subtle wrist movements that are related to disruptions in normal breathing rates during sleep. Every 30 days, Apple Watch analyzes the data on these disruptions and alerts users if they have signs of moderate to severe sleep apnea so they can talk to their doctor and potentially receive a diagnosis and treatment.“.
This feature is indeed intended to help with diagnosis for people who do not know they are affected. It is not intended for patients under treatment and remains less precise than a test carried out by a doctor but it would allow for better screening.By identifying irregular breathing rates during sleep, users around the world will be able to detect underdiagnosed and potentially serious conditions, such as sleep apnea.” explains Dr. Sairam Parthasarathy, professor and director of the Center for Sleep, Circadian, and Neurosciences at the University of Arizona in Tucson, Arizona.This is a major step in improving public health“.
Users can even export a PDF showing the times of their potential apneas and their three-month history of breathing disturbances, and share it with their doctor to discuss it based on more complete information.
An innovation that could save lives
Apple welcomed the development, saying it will help users better understand their health and take steps to seek professional help if signs of sleep apnea are detected. The feature will potentially allow doctors to monitor patients remotely.
With this new feature, Apple continues to push the boundaries of connected health. The FDA’s green light follows the same agency’s approval of Apple’s AirPods Pro 2 as over-the-counter hearing aids for users with mild to moderate hearing loss.