Posted ,
Reading 2 mins.
The National Agency for the Safety of Medicines and Health Products (ANSM) authorizes the use of phage therapy in a secure setting. This concerns adults and adolescents for the treatment of serious bone and osteo-articular infections caused by Staphylococcus aureus, when their vital or functional prognosis is at stake.
The National Agency for the Safety of Medicines and Health Products (ANSM) has just authorized the use of phage therapy thanks to the use of two bacteriophages in adults and adolescents, in the treatment of bone infections and severe osteo-articular staphylococcus aureus, when the vital or functional prognosis is engaged.
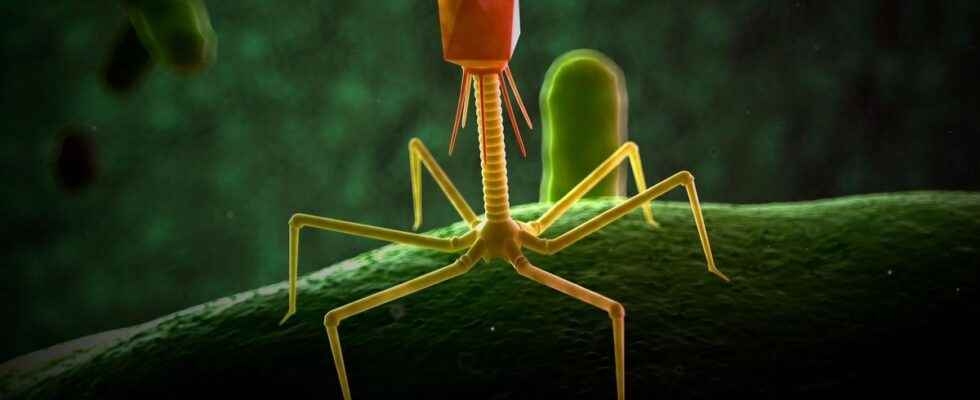
What is a bacteriophage?
A bacteriophage is a virus capable of infecting bacteria and causing their lysis, that is to say their destruction. Bacteriophages therefore represent a complementary approach to treat patients in therapeutic impasse in a context of antibiotic resistance. However, the ANSM specifies that “clinical trials must be conducted to demonstrate their effectiveness“.
Indeed, to date, no medicine containing bacteriophages has marketing authorization (AMM) either within the European Union or in the United States. However, “theClinical trials and compassionate access authorizations (AAC) can be set up because these bacteriophages are now produced and controlled according to quality standards equivalent to those required for all experimental drugs“, indicates the Agency.
In what context will they be used?
The ANSM authorizes the use of two anti-Staphylococcus aureus bacteriophages, called PP1493 and PP1815 developed by Pherycedes Pharma. Their use will apply to patients who cannot take part in a clinical trial, in particular the PhagoDAIR I clinical trial which should start soon, the only one authorized in France to date, even if others should follow.
Bacteriophages can therefore be used locally or by injection. in situ, in the hospital and within the framework of a compassionate access authorization, under the supervision of the ANSM. As a reminder, AACs are a “device which allows, by way of derogation, the use of medicinal products without MA or in a particular indication, in France, to treat serious or rare diseases when there is no appropriate treatment, when the patient cannot be included in a clinical trial and that the implementation of the treatment cannot be postponed“, specifies the health agency.
Bacteriophages are for the moment reserved for adult or adolescent patients who suffer from serious bone and osteo-articular infections due to Staphylococcus aureus, without the possibility of access to the therapeutic trial which is about to begin and in a situation of therapeutic impasse.
Necessary validation before the start of treatment
For each prescription request, validation by a collegial opinion given by a reference center for complex bone and joint infections (Crioac) must be requested.
These reference centers, spread throughout the national territory, are made up of multidisciplinary teams specialized in the management of bone and joint infections. They will be the guarantors of the therapeutic validation of applications for compassionate access to bacteriophages.