Published on
Updated
Reading 1 min.
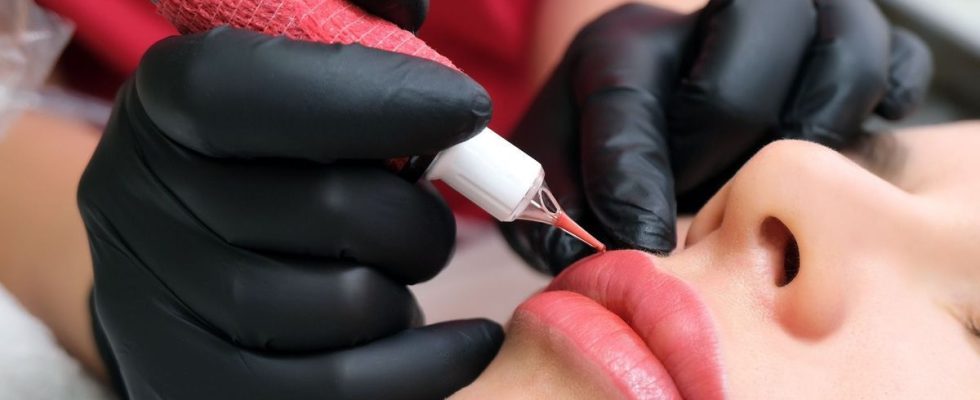
A mouth makeup product is being withdrawn from the market. The reason ? It would contain bacteria that could cause unwanted effects on your skin. We will explain the procedure to follow.
Certain batches of Vivid Koral Perma Blend Luxe, manufactured by Ink Projects LLC, have been removed from the market. Makeup ink, mainly used for the mouth, contains bacteria and certain batches are contaminated. For your health, it is recommended that you no longer use this product.
Tattoo ink: presence of bacteria
The product affected by the recall has the following information on the label:
- Product category: Cosmetics
- Product subcategory: Permanent makeup
- Brand: Perma Blend Luxe
- Model or reference: Vivid Koral Perma Blend Luxe tattoo ink
- Affected batches: LXVK 18726, LXVK 35103, LXVK 41136

What procedure should you follow if you have been tattooed with this product?
If you have received an injection of a Vivid Koral Perma Blend Luxe product, then it is recommended that you contact the professional who tattooed you to find out if they used one of the affected lots. In fact, it is normally possible to be kept informed because each establishment has traceability of the injections carried out.
In the event that the professional has used a product affected by the withdrawal, and you are the victim of adverse effects, it is recommended to consult a dermatologist or your treating physician.
The effects of the bacteria present in this product may manifest as redness, pimples, or any other skin reaction in the tattoo area. You are also invited to perform a declaration on the reporting portal of the National Agency for the Safety of Medicines and Health Products (ANSM)in order to report this product.
What procedure should you follow if you are a tattoo artist?
Professionals who have or use these lots of tattoo ink in their establishment are required to no longer do so and return them to the distributor.
It is also important to keep your customers who have received an injection of this product informed. If one of them notices an adverse effect, invite them to consult their doctor or a dermatologist.