Posted 1 day ago,
Reading 2 mins.
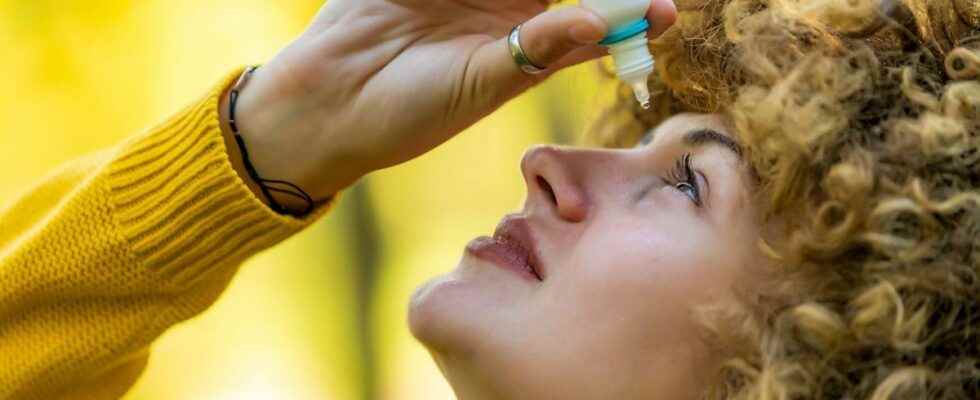
The first treatment for presbyopia – without glasses or corrective lenses – has been approved by US authorities. It is an eye drop called Vuity, based on pilocarpine. Explanations.
In France, 20 million people suffer from presbyopia, a progressive loss of the eye’s ability to “focus” on a nearby object. This visual disorder evolves gradually and stabilizes around the age of 60, which leads the patient to change his glasses every 2 to 3 years. To correct this anomaly, a new treatment has recently become available on prescription. It is a simple eye drop, developed by the pharmaceutical company Allergan, which improves near vision for six to ten hours.
An effective eye drop for several hours
With age, the elasticity of the lens decreases, limiting the eye’s ability to focus. An annoying visual defect (difficulty reading small print, etc.) which generally begins at the age of 40-45.
“Many Americans suffer from presbyopia … and adopt reading glasses or resort to workarounds – such as increasing the font size on their digital devices – to see up close.” said optometrist Selina McGee, a member of the American Academy of Optometry.
Except that for a few months, a new eye drop, formulated with pilocarpine (a substance already used in the treatment of glaucoma) and approved by the Food and Drug Administration (FDA), is now available on prescription.
This solution adapts to the pH of the tear film and reduces – in less than fifteen minutes – the size of the pupil to improve near and intermediate vision. Clearly, it offers perfectly clear vision for 6 to 10 hours. His price ? 80 dollars, or about 71 euros.
“We are thrilled to be able to bring this treatment, the first of its kind, to market sooner than expected for the millions of presbyopic Americans who could benefit from it.”said Jag Dosanjh, senior vice president of medical therapeutics at Allergan.
This important innovation in the field of health “reflects our commitment to advancing eye care”he added.
Consult an ophthalmologist online
Encouraging clinical trials
The US Medicines Agency authorized the eye drops in October 2021 following two phase 3 clinical studies with encouraging results.
Indeed, they showed that patients treated with Vuity were able to read at least three additional lines in near vision, compared to those treated with a placebo.
Furthermore, no serious side effects were observed although some participants reported headaches or red eyes. Finally, distance vision was not altered.
The laboratory specifies that these drops must “be used with caution when driving at night and practicing dangerous activities in low light conditions”.
Another clinical trial is underway to determine if the use of eye drops could be increased to twice daily.