Published on
Updated
Reading 3 mins.
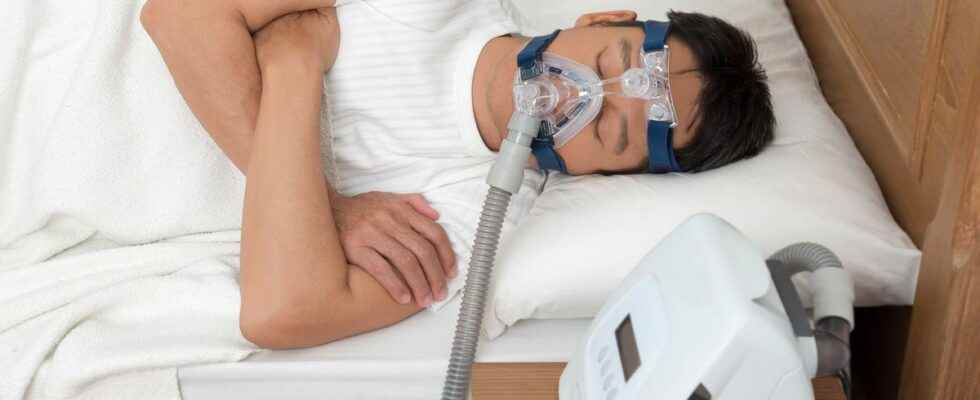
The affair broke out in the middle of 2021: respirators marketed by the giant Philips, used by patients suffering from sleep apnea, are withdrawn from the American market. In question: the polyurethane foam they contain, suspected of being carcinogenic. Our colleague, journalist Anne Jouan, agreed to give us the anonymous testimony of an ANSM director on the subject.
For almost a year and a half, Philips respirators have been making headlines. The devices are suspected of being dangerous to health, due to a foam that soundproofs the interior of the machines. The substance can degrade into chemical microparticles, inhaled by users and pose a risk of cancer.
A business that is progressing… slowly
Since then, the case has been progressing slowly and at least 128,000 French patients are still using these devices, while the Dutch giant promised a year ago to replace all the devices in circulation. At present, more than half of the respirators would have been replaced and Philips ensures that it will reach the replacement rate of 97% by the end of the year.
The Health Products Safety Agency takes up the case
In France, the National Agency for the Safety of Medicines and Health Products (ANSM) has taken up the case. At the beginning of 2022, the agency wanted to force Philips to replace defective devices quickly “according to a fixed schedule“.
In June, it organized a meeting with a committee of experts made up of representatives of associations of users of the health system and other medical specialists. To date, no reliable epidemiological study makes it possible to exclude any risk for patients, as underlined by the epidemiologists contacted by the ANSM, and no scientific study can establish a proven link between the use of the machines concerned. by recall and cancer.
Mismanagement by the health authorities?
Asked about the subject, Anne Jouan, journalist and co-author, with Professor Christian Riché, of the book-investigation entitled “Organized gang health“, published by Robert Laffont, who reveals behind the scenes of the Mediator affair, agrees to give us an exchange she had with a director of the ANSM, on October 5, on condition of anonymity.
Question from Anne Jouan: “In your opinion, has the Agency malfunctioned recently?”
Answer : “When I look at the management of Philips ventilators, the Agency’s slowness has exposed patients. There was no need for all this showmanship with this discussion on Youtube and all this repetitive communication making it look like the Agency is working on the subject while it is stalling.
The particles have been identified and analyzed by the FDA, the carcinogenic risk established. For the Agency, there were two things to do: replace the devices ASAP (as quickly as possible, editor’s note) and inform patients of the potential risks. Would the DG have accepted that his companion uses a Philips device for his sleep apnea? Would she have informed him that the particles can cause him a crab?
The risk of cancer is not proven? No, but that’s like saying that the Mediator has a cardiotoxic potential but that, in view of the evidence, the cardiotoxic risk is not proven. There is a risk that the particles are carcinogenic but we are waiting for this to be proven!
We do not tell patients what are the inhaled particles, what are the thresholds?
The ANSM gives information empty of information, it cannot make measurements to identify these particles instead of trusting Philips? Qualify the particles, quantify the quantities inhaled as well as the risk: there are CNRS labs, engineering schools perfectly capable of doing this. ANSM is waiting for the FDA to do so“.
A preliminary investigation opened
Last February, a collective action was launched by Maitre Lèguevacques, at the request of many patients and with the support of the French Federation of Associations and Friends of Respiratory Insufficient Patients (FFAAIR).
Two months later, in April 2022, Philips refused to communicate to the FFAAIR the exact composition of its polyurethane foam, the identity of its producer and the studies according to which the risk of cancer is not proven.
On June 20, 2022, the Paris Public Prosecutor’s Office opened a preliminary investigation for “endangering the life of others, aggravated deception and administration of harmful substances“and for its part, the federation has decided to summon Philips to appear on November 16 before the Nanterre court to force him to give his documents.