Posted ,
Reading 2 mins.
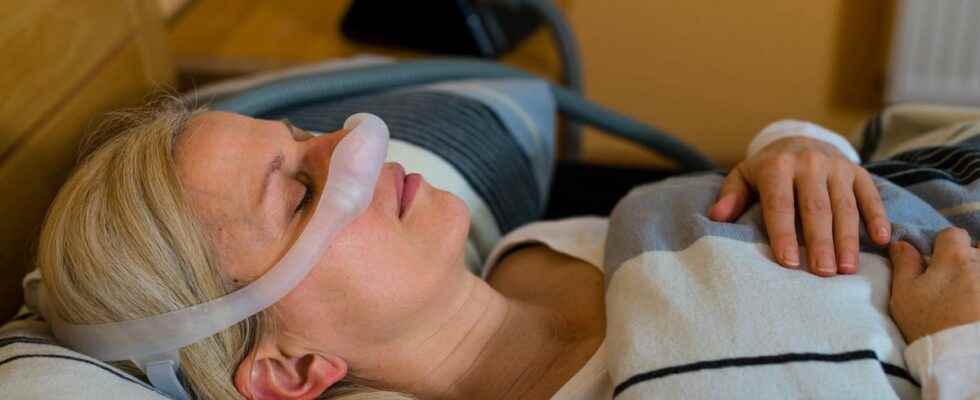
The Medicines Health Agency convened a committee of experts on June 8, 2022, to better understand the potential risks associated with the use of defective Philips respirators, the recall of which took place a year ago, on June 10. 2021. For patients, it is always a question of whether these respirators represent a danger to their health.
The case of the Philips ventilators is progressing step by step, but not fast enough for the taste of the patients. Yesterday, Wednesday June 8, the ANSM brought together a committee of experts bringing together the actors concerned for a hearing, which was broadcast live on the agency’s Youtube channel, to take stock of the situation.
Indeed, little has been done since the recall of defective respiratory devices by the giant Philips, a year ago, on June 10, 2021. As a reminder, these devices are suspected of releasing particles dangerous to health, coming from foams that go into the composition of the devices.
Objective: to carry out an inventory and issue an opinion
The will of the ANSM is twofold: through this committee, it wishes “carry out an inventory of the data available on the potential risks of using the Philips devices affected by the recall of June 10, 2021″“issue an opinion on these data and recommend, if necessary, additional studies” and “update its recommendations on maintaining the use of devices pending replacement.
For this, she brought all the stakeholders in this case around the table, namely: patient associations, representatives of Philips and qualified specialists because of their skills “in general medicine, pulmonology, toxicology, medical devices and epidemiology”.
Patients who are not more advanced
If the director general of the ANSM, Christelle Ratignier-Carbonneil, recognizes that the “patients are the first concerned because on the front line” because of the use of the machines, the feeling of the patients at the end of this meeting is mixed, as reported by Christian Truchot, member of the French Federation of respiratory insufficiency and representative of the main patient associations.
“There were people in high places at Philips who did not answer the questions asked, we still do not know the list of components of the foams of the devices “ he laments, also recalling that the current government, in particular the Ministry of Health, does not position itself more on the file. “The goal for us is to know if we can trust our device, whether it is Philips or other manufacturers, because there are more than a million devices of this type in circulation in the current time in France”. For Christian Truchot, it is important to know the truth because “we can change the device but not a life”.