Published on
Updated
Reading 3 mins.
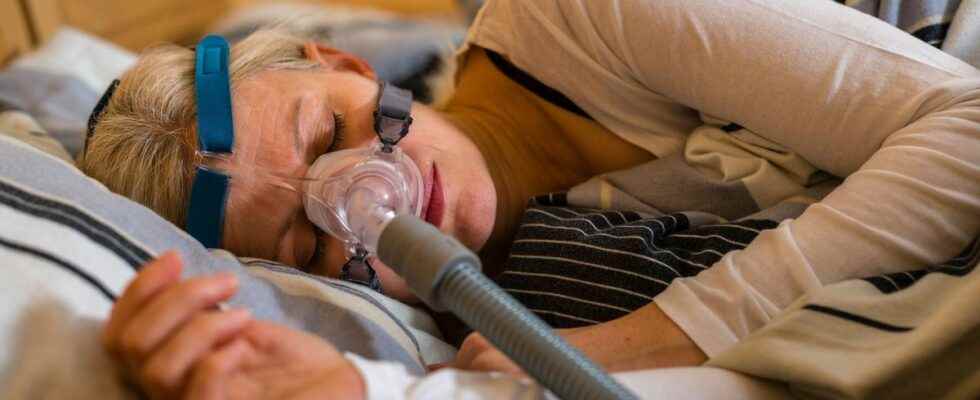
It is the National Agency for the Safety of Medicines (ANSM) which reports this alert from the company Philips. The manufacturer has indeed warned the agency of a “risk of electromagnetic interference between masks with magnets for ventilation devices and metallic implanted medical devices, such as pacemakers”.
After the case of respirators, still ongoing, Philips announces a new inconvenience related to its positive pressure ventilation equipment. This time it is a “risk of interference between the magnets present in the masks of these ventilation devices and the implanted metal medical devices”.
Possible malfunctions
The Philips company therefore informs the ANSM of these possible “interferences” between the masks containing the magnets and certain implanted medical devices. This can happen in the patient himself, when he uses the device, or when someone close to him is likely to find himself at a distance of less than 15 cm from the mask containing these magnets.
This proximity may therefore cause a malfunction in the implanted device, with potentially serious consequences, as in the case of a pacemaker, for example. At present, “14 cases have been reported worldwide, the majority in the United States”. In France, a case has been reported, “without consequence for the patient” according to the press release from the ANSM.
Which masks and devices are affected?
The six models of concerned masks are the following :
- The minimal contact Amara View face mask;
- The DreamWear face mask;
- The DreamWisp nasal mask with cushion on the nose;
- Wisp and Wisp Youth nasal masks;
- The 3100 NC/SP treatment mask.
About the implanted metallic medical devices likely to malfunction are the following:
- Pacemakers;
- Implantable cardioverter defibrillators (ICDs);
- Neurostimulators;
- Metal and magnetic implants/electrodes/valves placed in the upper limbs, torso or higher (i.e. neck and head);
- CSF (cerebrospinal fluid) shunts (e.g. ventriculoperitoneal shunt);
- Aneurysm clips;
- Embolization coils;
- Devices for interrupting the intravascular flow of intracranial aneurysms;
- Metal skull plates, screws, burr hole protectors and bone substitutes;
- Metallic splinters in the eye;
- Ocular implants (eg, glaucoma implants, retinal implants);
- Certain contact lenses containing metal;
- Implants to restore hearing or balance containing an implanted magnet (such as cochlear implants, implanted bone conduction hearing devices and brainstem hearing implants);
- Fixing systems for magnetic dental prostheses;
- Metal gastrointestinal clips;
- Metal stents (eg, aneurysm, coronary, tracheobronchial, biliary);
- Implantable ports and pumps (e.g. insulin pumps);
- Hypoglossal neurostimulators;
- Devices with the label “Not suitable for MRI” (magnetic resonance imaging);
- Metallic and magnetic implants that do not have an MRI-related label or whose safety in a magnetic field has not been evaluated.
What to do if you are affected?
Two cases arise: if you – or one of the members of your household – are concerned by one of these devices mentioned above, the ANSM recommends that you “promptly contact your doctor for advice and your home healthcare provider to replace your mask with a new mask without a magnet“.
While waiting for the change of material, the ANSM advises to “do not stop immediately“to use the mask, without having discussed it with the doctor.
If you do not wear this type of device, you can continue to use this mask, bearing in mind that “in general, the risk of interference with any metallic device, implanted or not, can be ruled out beyond 15 cm”.
Informed home health providers
Finally, the Agency indicates that home healthcare providers will be informed of the problem in order to discuss it with their patients and decide what action to take.
Finally, the ANSM recalls that “any device with a magnet is likely to cause interference with devices containing metals“.