Published on
Updated
Reading 2 min.
The Dutch giant Philips is obliged to stop the sale of its respiratory devices intended for patients treated for sleep apnea in the United States. The reason ? A suspected risk of cancer, linked to the polyurethane foam they contain. A decision that comes several years after the start of the brand’s defective respirator affair, which dates from 2021.
Philips will stop selling new breathing devices or other respiratory care products in the United States, about two and a half years after launching its massive recall of related products. The company agreed to this procedure as part of a consent decree it entered into with the U.S. Department of Justice, representing the Food and Drug Administration.
Positive pressure devices to combat sleep apnea
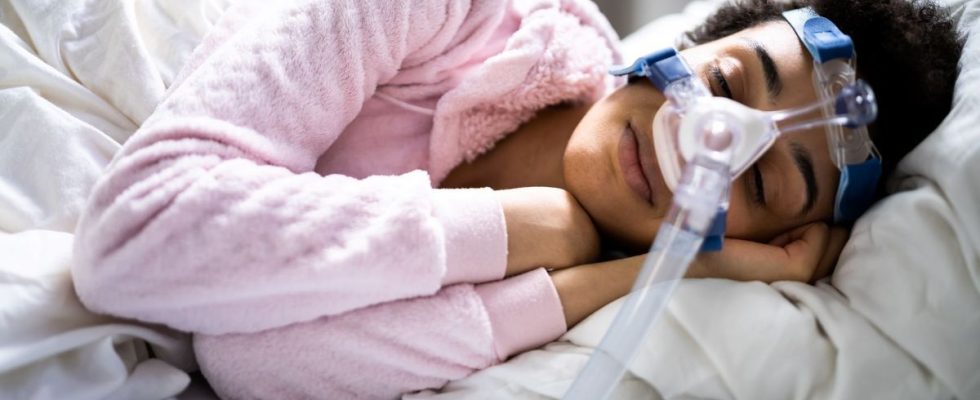
What are these respirators used for? In sleep apnea syndrome, patients experience breathing pauses during sleep, which repeat many times per night. Associated with snoring, this syndrome leads to poor quality of sleep at night, which has an impact on the day. Patients are then affected by alertness disorders and sometimes forced to take a nap to recover.
To help them, doctors equip them with positive pressure devices or CPAP, which diffuse oxygen, in order to breathe better at night. These ventilation devices consist of a respirator to which a mask is attached, which the patient attaches to their face before going to bed.
An increased risk of cancer because of Philips machines?
The problem with Philips fans comes from the sound-absorbing foam contained in the device. It is suspected of breaking down into fine particles, inhaled by patients and responsible for cancer, in the long term. Several recalls were issued by the company and the National Medicines Safety Agency forced the Dutch giant to change the devices supplied to patients.
A procedure which dragged on, especially since Philips is accused of knowing the problem with its devices since 2015, with some sources even estimating that the problem dates back to 2008. The company is now facing more than 700 lawsuits legal and the group experienced a net loss of 463 million euros in 2023.
Company CEO apologizes
In France, the National Agency for the Safety of Medicines and Health Products (ANSM) has carried out no less than 10 consultation meetings with Philips and user associations between June 2021 and March 2023, without succeeding in forcing the manufacturer to withdraw its respirators. In the end, the ANSM seizes the public prosecutor in April 2023. Last June, no less than 217 people filed a complaint before the Paris public health center for “deception, endangering the lives of others and administering harmful substances” in particular, against Philips regarding these respirators.
In just over two years, Philips has recalled 5.5 million of these devices out of a total of 15 million, including 350,000 in France and 1.5 million across Europe. But the American and European authorities believe that these recalls are not rapid enough, hence this new decision.
For his part, Roy Jakobs, CEO of Philips since October 2022, makes his mea culpa. “Resolving the impact of the Respironics recall on our patients and customers is a priority, and I apologize for the distress and concern caused” he said in a press release Monday January 29, 2024.