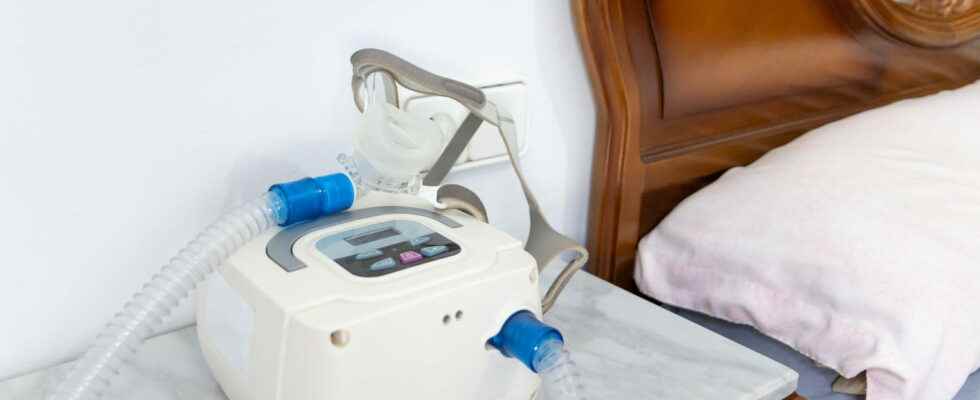
370,000 Philips brand sleep apnea devices have been recalled due to a potential risk of inhaling carcinogenic particles. An investigation has been opened at the Paris Public Prosecutor’s Office. Is this risk of cancer proven? Alert symptoms, list of affected models, what to do.
[Mis à jour le 8 septembre 2022 à 9h43] A Preliminary investigation was opened on June 20, 2022 by the health center of the Paris public prosecutor’s office, after 8 users of Philips sleep apnea ventilators complained. A dozen other complaints have been filed in the regions. This investigation was opened for “endangering the life of others“, “aggravated deception” and “administration of harmful substances”. What happened with these devices? Back to the facts.
Summary: What’s the deal with Philips ventilators?
► In July 2021, Philips released a security notification concerning CPAP devicesrespirators for patients with sleep apnea or requiring respiratory assistance at home. These devices help with breathing during the night. A risk of degradation of soundproofing foam (placed inside the device) in the form of particles that can be ingested or inhaled by the user. Problem: degraded foam could form potentially carcinogenic volatile organic compounds (VOCs).
► On July 8, 2021, the Medicines Agency has brought together a committee of experts made up of representatives of associations of users of the health system as well as personalities qualified by virtue of their skills in general medicine, pneumology, toxicology, medical devices and epidemiology, in order to better understand the potential risks associated with the use of these defective devices. To date, the ANSM received nearly 300,000 reports of adverse effects.
► As a precaution, all the devices concerned (370,000 models in France) have been recalled, indicates theMedicines Agency in November 2021. These devices were supposed to be replaced however, this replacement has been delayed and some patients have continued to use the devices in question, not having been made aware of these risks. To date, Philips had replaced only a third of the defective devices.
► February 7, 2022L’ANSM which held a new exchange meeting, wanted to compel Philips to speed up the replacement of the machines and that the home care providers inform the patients concerned, by the making a health policy decision.
► February 11, 2022Philips reported his disagreement in a statementconsidering “that the ANSM’s health policy decision is unjustified and is studying the possibility of appealing against this decision as soon as possible“.
What is the risk of cancer with defective Philips appliances?
Based on the first data available which was still limited at that time, Philips, as an extreme precaution and for the sake of transparency, announced potential carcinogenic risks related to the use of some of its devices. Since then, additional toxicological tests and analyzes have been carried out by certified testing laboratories and a qualified third-party expert. As of December 2021, these tests revealed reassuring results on VOC emissions 1st generation DreamStation devices. Similarly, over an identical period, a completely independent Canadian study, published in the American Journal of Respiratory and Critical Care Medicine and carried out on approximately 6,900 patients with OSAS who used a PAP device between 2012 and 2020, of which approximately 1,200 users of Philips CPAP devices, did not show a higher risk of cancer in the latter, compared to patients using a CPAP device from another manufacturer or to patients with OSAS without a device.
Which Philips devices are affected by the recall?
Devices manufactured after April 26, 2021 and those of other brands are not affected.
Philips released the list of models concerned (manufactured before April 26, 2021) by the recall:
- ventilators with life support (Trilogy100, Trilogy200) used at home, in hospitals, and in healthcare facilities for ventilator-dependent patients;
- ventilators without life support (BiPAP autoSV (DreamStation, Advanced, PR1/SystemOne, C-series), BiPAP S/T and AVAPS ( DreamStation, PR1, C-series), Omnilab Advanced +, BiPAP A30, BiPAP A40, BiPAP SOH) used at home for patients suffering in particular from COPD, hypoventilation obesity syndrome, kyphoscoliosis and neuromuscular pathology;
- continuous positive airway pressure devices (CPAP) (REMstar Pro, Auto, Expert (DreamStation, PR1/SystemOne, Q-series), BiPAP Auto, DreamStation Go) used at home mainly to treat sleep apnea syndrome
Please note, devices manufactured after April 26, 2021 and those of other brands are not affected.. To find out the model of your device, refer to the instructions on it. If the date of manufacture is not specified, contact your home care provider or the doctor who prescribed it. This information also appears in your tracking book, together with the serial number of your device.
Therefore, Philips Respironics is bound by our health policy decision to expedite the replacement of machines as follows:
- the replacement with service providers or the repair of 75% of the devices concerned in June 2022;
- replacement with service providers or repair of 100% of devices in December 2022
Philips Respironics is also required to:
- send the ANSM a monthly progress report on the schedule;
- to set up an epidemiological study to assess the risk of cancer potentially induced by exposure to the ventilation equipment concerned, the preliminary results of which must be sent to the ANSM within one year at the latest.
What are the warning symptoms with Philips appliances?

In the event of exposure to degraded foam particles, the risks are:
- Of the irritation (skin, eyes and respiratory tract)
- Of the cough,
- A chest pressure
- A sinus infection
- Of the headache or some dizziness
- Of the’asthma
- Adverse effects on other organs (for example, kidneys and liver)
- Of the nausea or vomiting
- Risks of toxicity and carcinogenic risks : “in view of the data transmitted by Philips, a carcinogenic risk after long-term exposure of the devices concerned cannot be excluded“, indicates theANSM.
What should be done for users of Philips respirators?
Pending the results of its investigations, the ANSM has told patients who use these machines what to do:
- Do not stop your treatmentregardless of the type of device used. Stopping treatment presents a proven short-term risk, for example aggravation of respiratory failure. According to the first data available, the risk of cancer linked to the use of these devices is not proven, specifies the ANSM.
- Your pulmonologist or home healthcare provider has contacted you or will contact you to arrange for the repair or replacement of your equipment, depending on the availability of equipment.
- In case of headache, irritation (skin, eyes, respiratory tract), inflammatory reactions, cough, chest pressure, asthma and sinus infectionor other symptoms that may be related to the use of the device, contact your doctor.
- In the event of such undesirable effects, we also invite you to A declaration on the reporting portalspecifying the full serial number of the device (your doctor can help you with this statement).
For their part, the doctors of the French Language Pneumology Society (SPLF) also advise continuing to use the respirator in their possession. The risk-benefit balance being favourable.