Published on
updated on
Reading 3 min.
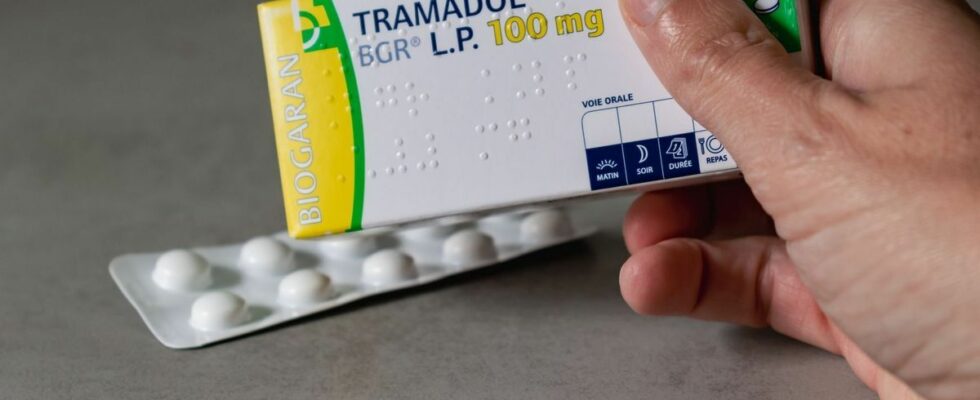
From December 1, tramadol and codeine, two drugs widely used for pain but with a high risk of dependence, can now only be dispensed with a secure prescription. This measure aims to combat the risks of opioid addiction.
This decision, announced by the National Medicines Safety Agency (ANSM), comes in response to the worrying rise in abuse and dependence linked to these medications, often compared to hard drugs because of their effects.
Why this new regulation?
The decision to impose tamper-proof prescriptions for tramadol and codeine was motivated by an alarming increase in cases of dependence, overuse, and even overdoses associated with these two opioids. In 2021, an ANSM study revealed a constant increase in hospitalizations and deaths linked to the consumption of these medications. This new regulation aims to limit the risks of fraudulent prescriptions and to better regulate the dispensing of these substances, in particular by reducing the possibilities of improper obtaining through falsified prescriptions.
Tramadol, in particular, is often prescribed for moderate to severe pain, but it is also known for its psychotropic effects that make it particularly addictive. According to the ANSM, around 13 million boxes of tramadol are sold each year in France, placing this drug among the most prescribed in the country. Codeine, for its part, although less powerful, is often used in combination with other painkillers and also has a strong addictive potential.
Unfalsifiable prescriptions to better regulate delivery
In order to reduce these risks, the ANSM has already taken several measures:
- Since 2017, all medications containing codeine are subject to a medical prescription;
- In April 2020, the maximum prescription duration for medicines containing tramadol is reduced to 12 weeks (three months);
- Manufacturers marketing medicines containing tramadol have been asked to place on the market boxes containing fewer tablets, suitable for short-term treatments, in addition to the boxes already available.
But the ANSM admits that these measures have not made it possible to sufficiently reduce the misuse associated with these drugs. This is why it is taking new measures with the mandatory use of secure prescriptions for these products and an alignment of prescription periods reduced to a maximum of 3 months for all these medications.
Secure prescriptions, already used for prescribing other controlled substances such as narcotics, have several features aimed at guaranteeing their authenticity. They incorporate security features such as holograms, unique numbers, and a watermark, making them extremely difficult to tamper with. This measure is also supported by pharmacists and doctors, who see it as an effective way to strengthen control over opioid consumption in France. This measure should make it possible to reduce diversions while guaranteeing more rigorous monitoring of patients.
At the same time, the Agency declares that it is working “the implementation of additional measures to better inform patients about the risks of dependence and overdose linked to these medications”. The Agency even specifies that it plans to ask laboratories to affix “warning notices on medicine boxes containing tramadol or codeine“.
A response to the opioid crisis
This decision comes as France, like other countries, faces an opioid crisis. Although less affected than the United States, where opioid-related overdoses have claimed hundreds of thousands of victims, France is experiencing a continued increase in cases of addiction and accidental deaths. In 2020, 400 deaths linked to opioid consumption were recorded in France, a statistic which pushed the authorities to strengthen prevention measures.
Reminders for the proper use of medications containing tramadol or codeine according to the ANSM
- Respect the dosage, duration of treatment and interval between doses;
- Do not stop your treatment suddenly to avoid side effects related to withdrawal. Your doctor or pharmacist will tell you how to stop it gradually;
- Never offer your treatment to anyone you know, even if they seem to you to have symptoms similar to yours;
- If a person (child or adult) has ingested tramadol or codeine that was not intended for them, immediately contact a poison control center or emergency service: 15 (Samu), 18 (fire brigade) or 112 (all emergencies: medical, fire, security);
- For people at risk of overdose who have a naloxone kit (opioid antidote): if the person is drowsy, call for help, administer naloxone, and keep them awake until help arrives. relief.
If you experience any side effects from taking these treatments, consult your doctor and report them to the reporting portal.