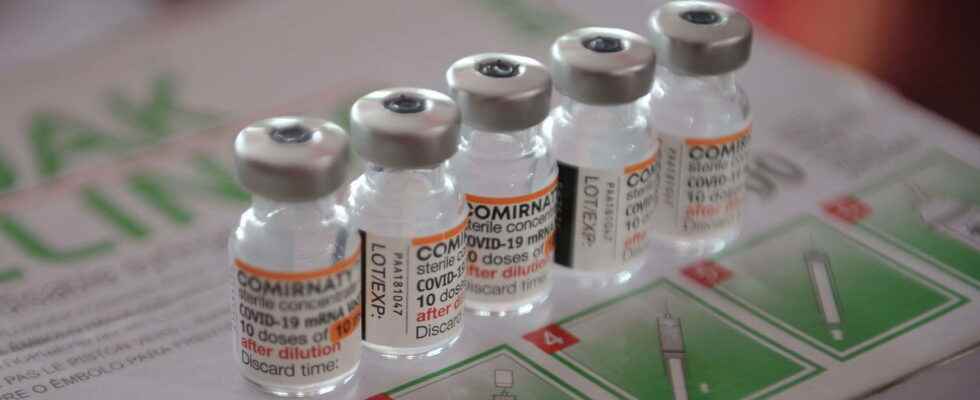
COVID VACCINE. New Pfizer and Moderna vaccines are approved by the EMA on September 1, 2022. These are bivalent vaccines capable of better protecting against Covid-19 and all Omicron variants.
[Mis à jour le 1er septembre 2022 à 15h28] Two new vaccines against Covid-19 were approved by the European Medicines Agency (EMA) this Thursday, September 1, 2022. At first glance, the arrival of the products may seem late, but the bivalent vaccines signed Pfizer-BioNTech and Moderna present a formula better suited against Omicron variants. And for good reason, they combine the vaccine developed against the spike protein of the Covid-19 stem cell and that against the protein of the BA.1 variant, the first mutation of Omicron. The new messenger RNA vaccines therefore offer “better immune response” according to Morgane Bomsel, immunologist at the Institut Cochin contacted by the Parisianthe protection would also be more effective on Omicron and all its variants including B4 and B5.
The two bivalent vaccines therefore seem to be ideal for use during the vaccination campaign planned for autumn 2022, in particular for people who have to use a 4th dose of vaccine. The youngest from Pfizer and Moderna must also and above all serve to prevent if a new epidemic wave breaks out in the coming weeks. The president of the Technical Vaccination Commission, Élisabeth Bouvet, interviewed by Point explains in particular that if Covid-19 were to circulate again, bivalent vaccines “would make it possible to expand the repertoire of antibodies available to our immune system and this would strengthen our defenses”. Enough to avoid contamination, serious cases and admissions to intensive care.
When will the new Pfizer and Moderna bivalent vaccines be available?
The European Medicines Agency gave the green light to the new Pfizer and Moderna vaccines on September 1, but the products have yet to pass the Haute Autorité de Santé. The French gendarme must give his opinion on September 5 but should, barring a big surprise, approve the bivalent vaccines. It will then remain to order deliveries of Pfizer and Moderna products. On the side of Moderna, we assure “to be ready” and Sandra Fournier, the general manager of the French branch of the laboratory, assured the Parisian to be able to “start delivering [le vaccin, NDLR] quickly” so that it is available this fall. And the director adds that her vaccine “will play an essential role in the booster vaccination campaign”
Learn more
During the Covid-19 epidemic, the availability of vaccines was one of the challenges and the main products arrived in the course of 2021, the major pharmaceutical companies having made it a priority. It was the American Pfizer, allied with the German BioNTech, which was the first to put a vaccine on the market at the end of 2020, but many other players then responded to the health crisis with other products.
The main types of Covid vaccines
| Laboratory – vaccine name | Efficiency | Functioning | Availablity | EU ordered doses |
| Pfizer / BioNTech | 95% | RNA vaccine | Available in EU | 300 million |
| Moderna | 94.5% | RNA vaccine | Available in EU | 160 million |
| Astra Zeneca | 70% | Viral vector vaccine | Available in EU | 400 million |
| Janssen / Johnson & Johnson | 66% | Viral vector vaccine | Available in EU | 400 million |
| Sputnik V | 95% | Viral vector vaccine | Available outside EU | 0 |
| Sinovac | 50% | Inactivated vaccine | Available outside EU | 0 |
| Novavax | 96% | protein vaccine | Spring 2021 | 0 |
| Curevac | Evaluation in progress | RNA vaccine | Not disclosed | 405 million |
| Valneva | Evaluation in progress | Inactivated vaccine | Not disclosed | 0 |
The effectiveness of Covid vaccines
The Pfizer vaccine has an effectiveness rate of around 95%, that is to say that it allows in 95% of cases to prevent Covid-19 from developing a serious form of the disease on the infected person. This assessment was first established at the end of a clinical trial on nearly 40,000 people, carried out by the Pfizer laboratory. The results were then made public in a press release, then on a dedicated page of the Pfizer site. A study conducted on mass vaccination in Israel, where more than half of the population was vaccinated at the beginning of March 2021, has also established that with the Pfizer vaccine, serious forms of the disease in the event of infection do not occur. in 92% of cases. According to the results of this study published in The New England Journal of Medicine, symptomatic infections after the second dose are significantly reduced in 94% of cases after a second injection. Another scientific study, published in March 2021 by the UK Governmentshows that the Pfizer vaccine “significantly” reduces severe cases in people over 80.
As for the efficacy of the vaccine on the variants, another study published in February 2021 in the journal Nature gives elements demonstrating that the serum is effective, to a lesser extent admittedly, in limiting its serious effects. And the vaccine loses a little more effectiveness against the Indian variant according to a new study conducted by researchers from the Institut Pasteur. “The sera of patients who have had Covid-19 and collected up to 12 months after symptoms as well as people who have received the Pfizer vaccine remain neutralizing, but are 3 to 6 times less potent against the Indian variant compared to the English variant. “, explains Olivier Schwartz. The scientist, however, wants to put the figures into perspective: “The Pfizer vaccine is probably protective” even with “a slightly reduced effectiveness”.
The AstraZeneca vaccine was first shown to be 70% effective in a press release from the laboratoryfollowing phase 3 clinical trials. The British government, which has launched a vast vaccination campaign with this product, at a very sustained pace, published another study, on March 1, 2021, according to which a dose of the vaccine reduces the symptoms of Covid in 60 to 70% of cases. Otherwise, a preprint in The Lancet, posted on February 4, 2021, tends to show significant efficacy of the AstraZeneca vaccine against the British variant. The Haute Autorité de Santé, in France, has recognized the effectiveness of the AstraZeneca vaccine against symptomatic forms of the coronavirus, recommending in the first place “preferentially this vaccine in those under 65”. The HAS then considered, on March 2, 2021, that “the place in the vaccine strategy of the AstraZeneca vaccine” could “be extended to people over the age of 65”. Then, the situation reversed on March 19: after a three-day suspension of AstraZeneca in France, decreed by Emmanuel Macron, the HAS gave the green light to the use of the vaccine but recommended it only to people aged over 55 years old. On Monday March 22, the laboratory attacks again and indicates that its vaccine was 79% effective against Covid-19 and does not increase the risk of clots, after the clinical trial conducted in the United States. Furthermore, he reaffirms that the serum is 80% effective for people over the age of 65.
Regarding the variants, in particular the Indian mutation, in their latest study, researchers from the Institut Pasteur were only able to assess the effectiveness of the vaccine after an injection. The 12-week delay between the two injections and the later use of the product do not allow obtaining enough volunteers to study the efficacy in the case of a complete vaccination schedule. The study finds in view of laboratory tests that while AstraZeneca is effective against the English variant, it “works very little against the Indian and South African variants”. A single dose of the vaccine therefore proves to be “little or not at all effective” on the mutation explains Olivier Schwartz.
Read also
The Moderna vaccine presented in a first official press release an effectiveness of 94%, at the end of a so-called phase 3 clinical trial. France, through the High Authority for Health, welcomed in an official notice “the results of clinical studies which indicate a vaccine efficacy of the vaccine developed by Moderna on the reduction in the number of symptomatic Covid-19 cases, including in patients over 65 years of age”. The EMA has for its part published a long report concluding with its authorization on European soil. In addition, Moderna assures that its serum remains very effective against the English variant, but recognizes in a press release dated January 25 weaknesses against the South African variant.
The Janssen vaccine is considered by the American health authorities, in an official notice of February 24, 2021, as effective against severe forms of Covid-19 in a range of 82 to 86%. A result concluded at the end of an independent clinical trial on 43,000 participants. According to this study, the vaccine is effective against symptomatic forms in 66% of cases and 57% against the South African variant. On March 12, 2021, the French High Authority for Health in turn issued an official notice, welcoming “the satisfactory efficacy and tolerance profile of this vaccine”. It should be noted, however, that following the publication of a notice of the HAS on February 21, 2022, the French executive decided to suspend injections with the Janssen vaccine, reserving it only for people at risk of having a severe form of Covid-19 and who have a contraindication to administration of a messenger RNA vaccine. In its opinion, the HAS underlines the “slight increase in the risk of myocardial infarction”, “in the two weeks following” the injection of Janssen in adults under 75 years of age.
If the vaccines of the different laboratories have the same objective, namely to immunize against Covid-19, not all of them use the same technique to do this. There are various functions:
- Pfizer and Moderna both use the messenger RNA technique. The latter aims to give the body the genetic information necessary to trigger protection against the virus. The vaccine’s messenger RNA inserts and takes control of this machinery to produce a specific antigen of the coronavirus: the “spike” of the coronavirus, its so recognizable tip which is on its surface and allows it to attach to human cells to penetrate them. This spike, harmless in itself, will then be detected by the immune system which will produce antibodies, and these antibodies will remain. This method, never used for humans, could cause some complications, in particular on the conservation of the vaccine, which would be done at very low temperatures.
- Astra ZenecaSputnik and Janssen (Johnson&Johnson) use the viral vector technique. Unlike the original process, this one, relatively recent in terms of the history of vaccination, does not inject the infectious agent of a pathology, in this case Covid-19, in live or inactive form. . Instead, humans are injected with a “harmless vector containing one or more genes of the infectious agent encoding antigens capable of being recognized by the immune system”, as indicated by the site of the federation for brain research. There are two types of viral vectors. There are those which are said to be integrative, when the DNA of the viral vector integrates into the DNA of the host and the non-integrative, when the therapeutic gene remains in the cell without integrating into the host genome. (Source: Inserm). Several well-known vaccines use this technique, such as hepatitis B serums.
- Sanofi, the French vaccine, uses the technique based on recombinant protein. It is a protein produced by a cell whose genome has been transformed by genetic recombination.