Published: Less than 30 min ago
The European Commission gives the green light for a preventive treatment against the virus that causes the respiratory infection bronchiolitis in infants. Astra Zeneca is one of the companies behind the drug.
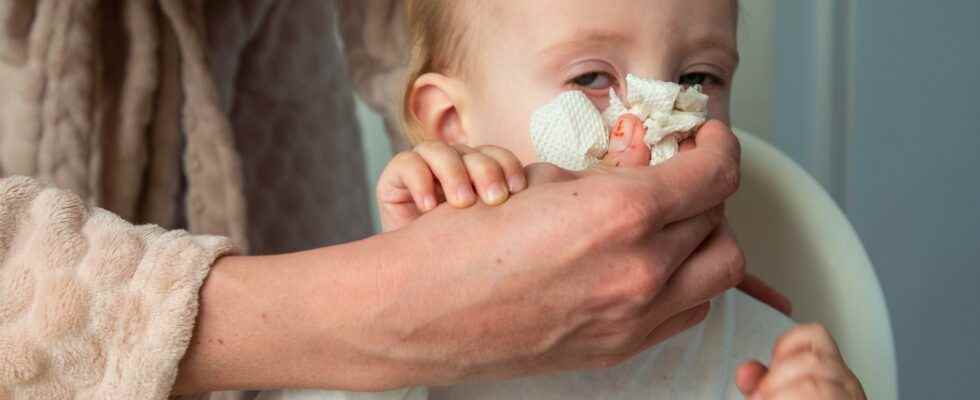
Bronchiolitis is an infection of the lower respiratory tract in children aged 0-2 years that is normally mild, but in some cases can make infants seriously ill. The RS virus, which is common during the winter months, causes 70-90 percent of cases of bronchiolitis.
Astra Zeneca and France’s Sanofi say their approved drug nirsevimab, marketed under the name Beyfortus, is the first to prevent severe disease caused by the RS virus in infants.
Although not a vaccine, nirsevimab has the same purpose: to provide protection against the RS virus through a single dose. The injection is a monoclonal antibody treatment that arms the body to fight the disease, unlike a vaccine that triggers the immune system’s own antibodies.
Several countries have seen an increase in cases of bronchiolitis among infants in recent years, with France reporting the highest rate of hospitalizations for the disease in years. However, Beyfortus will only be available next winter.
There is not yet an RS vaccine available on the market, but several are in development. Earlier this week, American pharmaceutical giant Pfizer announced positive results from a phase 3 study, paving the way for future approval. The vaccine was found to be 82 percent effective in preventing serious illness from RSV during the first three months of a child’s life.