Posted ,
Reading 3 mins.
The National Agency for the Safety of Medicines and Health Products (ANSM) continues its work of reinforced surveillance of vaccines against Covid-19. It brought together representatives of patient associations and health professionals for a situation update on the many menstrual cycle disorders reported by some women after vaccination.
The ANSM continues its monitoring work on vaccines against Covid-19, more particularly the adverse side effects declared by many women concerning their menstrual cycle.
A videoconference with all stakeholders
For this, it brought together France Assos Santé, Endomind, EndoFrance, the Ouestmoncycle collective, regional pharmacovigilance centers (CRPV), the National Federation of Medical Gynecology Colleges (FNCGM), the National College of Medical Gynecology Teachers (CNEGM) , the National Council of the Order of Midwives (CNOSF) and the College of General Medicine (CMG), in order to discuss the issue.
“Careful” monitoring
Indeed, many women have seen their menstrual cycle disrupted after being vaccinated against Covid-19. They complain of various disorders: severe abdominal or pelvic pain, irregularities in the menstrual cycle, which can affect its frequency and the intensity of bleeding, but also irregular, painful, excessively heavy, excessively prolonged or absent periods.
It is also possible that bleeding occurs between two cycles. In women with endometriosis, some have seen a reactivation of their painful symptoms when the disease was well controlled so far. Some postmenopausal women have noticed abnormal bleeding and finally, in some women, this bleeding has resulted in the removal of the uterus.
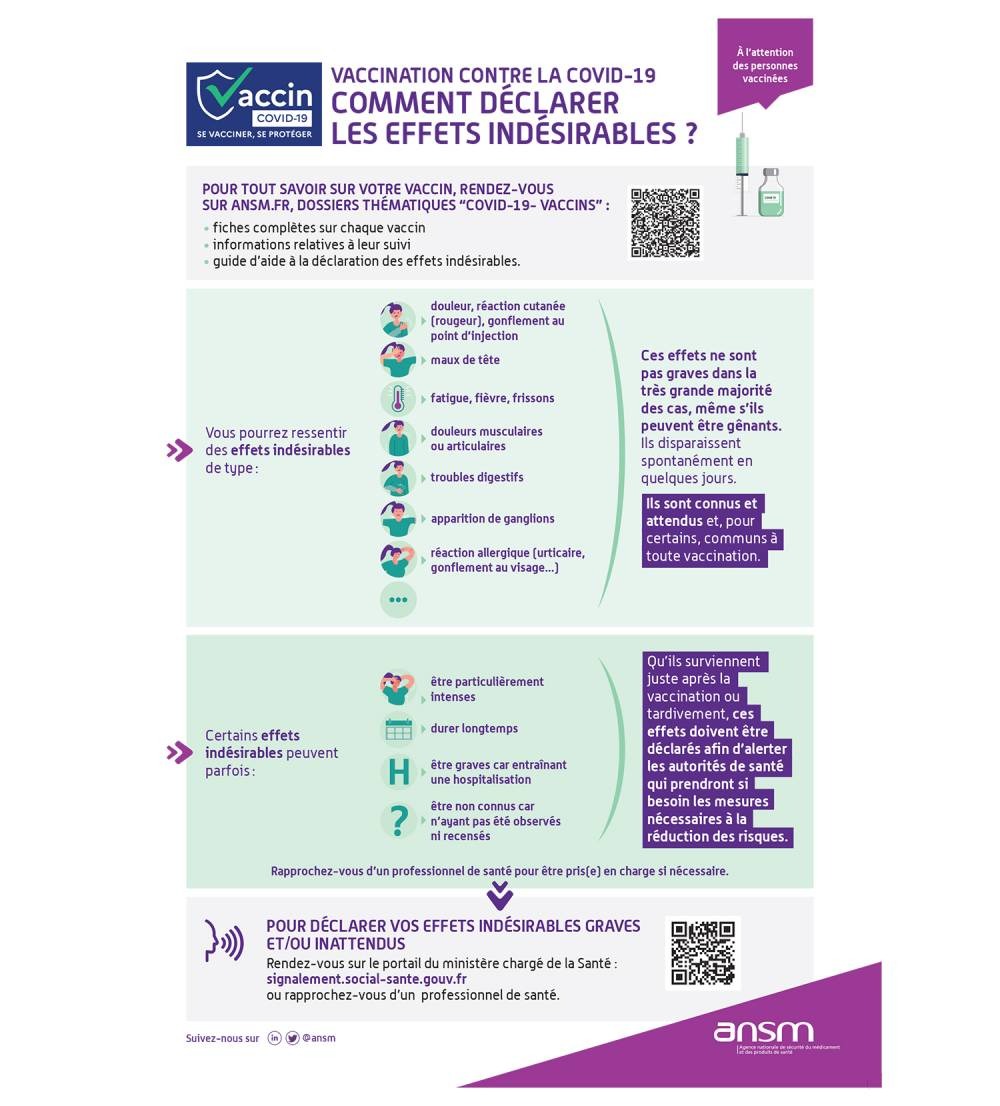
The Importance of Disorder Reporting
“Menstrual disorders reported after vaccination with an mRNA vaccine have been carefully monitored at national (ANSM/CRPV) and European (EMA) level since their detection. underlines the ANSM in its press release. However, to gather as much data as possible on the issue, the ANSM recalls the importance of declaring the changes felt by the women concerned. She invites women who have one or more disorders of their cycle to:
- Consult their doctor, to be listened to and undergo additional examinations if necessary.
- Declare their situation on the portal of the Ministry of Health: signalement.social-sante.gouv.fr.
Recommendations for professionals too
The ANSM also recalls the course of action for health professionals faced with a patient’s statement on this subject. Depending on the skills of the professional, he will have to:
- If the patient is taking hormonal treatment, check that there has been no poor compliance or vomiting which could be the cause of an interruption in taking the treatment;
- If the patient is not taking hormonal treatment or if there has been no interruption of treatment, check that it is not an acute symptomatology or a possible pregnancy that would explain a delay in menstruation or repeated bleeding.
Finally, the ANSM reminds health professionals to “keep in mind the possibility that the patient develops a gynecological disease (polycystic ovary syndrome, hyperprolactinemia, adenomyosis, etc.) concomitantly with vaccination”. It invites them to launch additional investigations if the symptoms persist and to declare on the portal of the Ministry of Health “any serious or unexpected side effects“.
Consult a GP online
No link with vaccination officially established
In practice, the health professionals interviewed report that they find “few serious cases of menstrual disorders” and that the majority of reported adverse reactions were “non-serious, of short duration and self-limiting”. They further note that serious cases remain “difficult to analyze because poorly documented”.
Furthermore, the Pharmacovigilance Committee (PRAC) of the European Medicines Agency (EMA) is currently reassessing the link between these menstrual disorders and the Spikevax (Moderna) and Comirnaty (Pfizer-BioNTech) vaccines and its first conclusions of June 2022 indicate that “there is insufficient evidence to establish a link between the two“.
For Dr. Odile Bagot, obstetrician-gynecologist and member of the Doctissimo expert committee, “the vaccine cannot be implicated in a strong and sure way, even if its implication is not impossible. What should be remembered is that these disorders are in most cases mild and disappear spontaneously. On the other hand, if they persist, it is because a gynecological pathology has declared itself and it is absolutely necessary to look for it” warns the specialist.
Finally, other avenues, such as reactogenicity following vaccination, significant stress or certain underlying elements (pregnancy, contraceptive treatment, gynecological disease, etc.) have been mentioned by the ANSM to provide explanations to the women concerned. .