Published on
Updated
Reading 3 min.
A new type of gene therapy has transformed the lives of people with a rare genetic disorder that causes painful and unpredictable bouts of edema. This success in a single injection against hereditary angioedema opens new perspectives against other genetic diseases.
A disease characterized by the occurrence of painful edema
Hereditary angioedema is a genetic disease, which results from an alteration of a gene located on chromosome 11. This disease is called autosomal dominant, that is to say it can be transmitted as long as Only one of the two parents carries the disease. There is a 50% chance that a person with HAE will pass it on to their child. However, in a quarter of cases, the gene mutation is spontaneous, and the parents are not carriers of the disease.
The disease manifests itself by edema, namely swelling of the tissues caused by the presence of an abnormal quantity of fluid. These circumscribed edemas generally persist between 2 and 5 days (transient edemas) and do not itch. Any part of the body can be affected, including the stomach, hands, feet, face and throat (laryngeal edema is particularly dangerous because it can lead to asphyxiation).
Potentially serious and very disabling crises
The disease manifests itself in attacks, the frequency and severity of which vary. Certain episodes can be particularly painful, for example in cases of abdominal edema. The majority of attacks occur for no apparent reason (stress, infections, cold, hormonal changes could, however, increase the intensity of attacks).
Today, the treatment of seizures is based on the subcutaneous administration of a bradykinin B2 receptor antagonist, or the intravenous administration of C1-INH concentrate. But today, a discovery could revolutionize the treatment of this disease.
Effective gene therapy in a single injection!
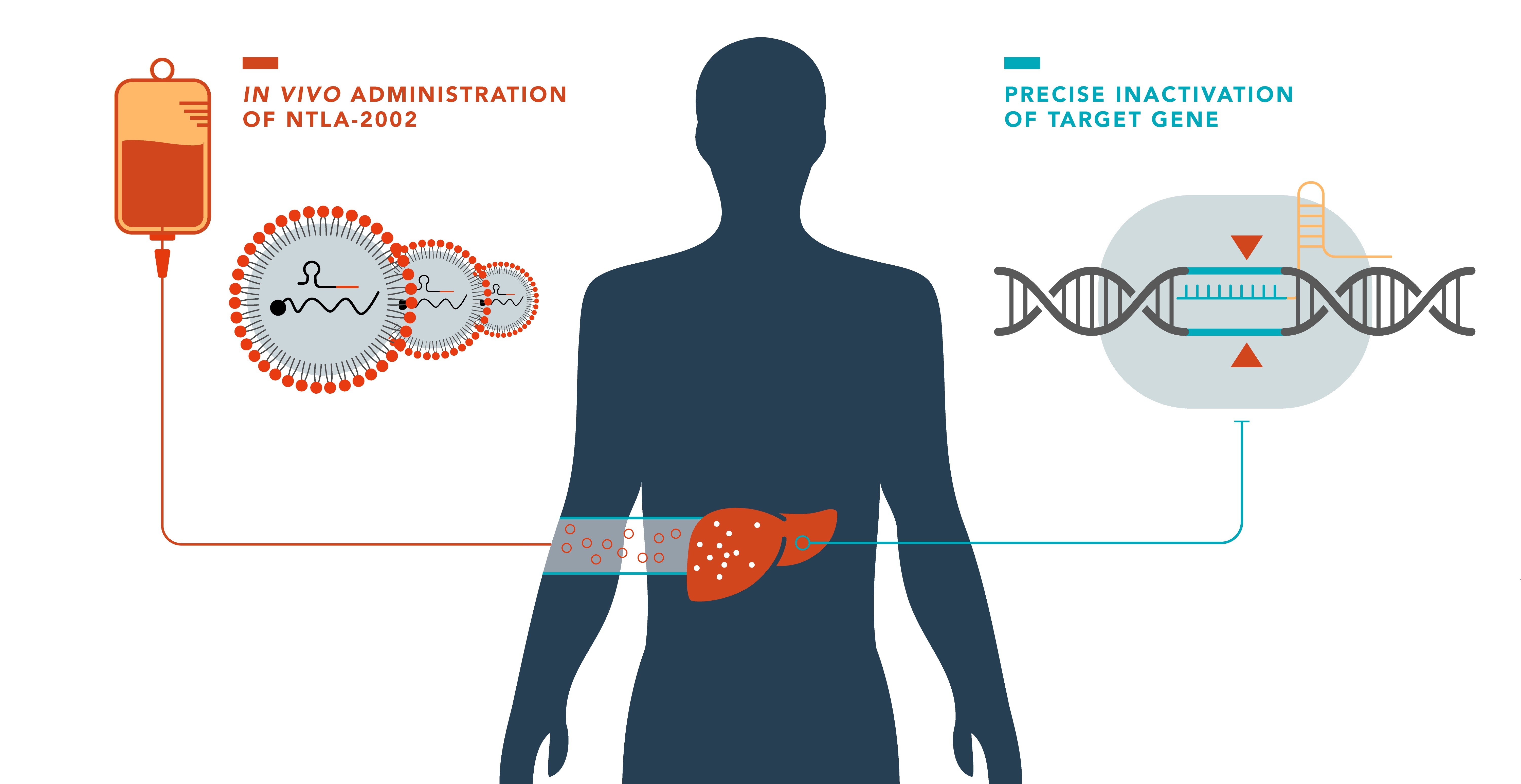
Researchers from the University of Auckland, University of Amsterdam Medical Center and Cambridge University Hospitals have successfully treated more than ten patients with CRISPR/Cas9 therapy, with interim results just published in the prestigious magazine The New England Journal of Medicine.
The experimental therapy, called NTLA-2002, uses CRISPR/Cas9 technology in vivo to target the KLKB1 gene, responsible for the production of kallikrein B1, involved in the occurrence of angioedema (swelling) attacks. In the phase 1 study, the single infusion, which took place over two to four hours under clinical monitoring starting in late 2021, revealed no serious or lasting side effects. The trial demonstrated a dose-dependent reduction in total plasma kallikrein protein, with reductions up to 95%!
“It appears that the single dose treatment will permanently cure the very debilitating symptoms of my patients with hereditary angioedema” says Dr Hilary Longhurst, lead author.
Patients in the initial study will be followed for an additional 15 years to continue to evaluate long-term safety and effectiveness. A larger, more robust, double-blind, placebo-controlled phase two trial is underway and a phase 3 trial is expected to start in the second half of 2024.
A “medical magic wand” for patients
According to the lead researcher, these patients saw their lives transformed after just one injection. Among them, Judy Knox, a New Zealand patient, declared: “Having undergone CRISPR/Cas9 therapy was like a medical magic wand, it changed my life“. Today, she is off her medication and feels like she has a new life. The number of seizures among her patients has dropped by 95%!
“We have never been closer to the ultimate treatment goal of normalizing the lives of hereditary angioedema patients and providing total disease control” says Dr. Cohn.
Important prospects linked to Crispr/Cas9
The studies were funded by the American company Intellia Therapeutics. So far, the only CRISPR therapy approved in the United States is CASGEVY, which is for sickle cell disease and beta thalassemia. The difference is that CASGEVY is an ex vivo CRISPR therapy, in which cells are taken from the patient and edited outside the body, then reinjected, while NTLA-2002 is an in vivo CRISPR therapy, in which the editing Targeted genes occur directly in the body.
CRISPR technologies could potentially be used to develop treatments for a wide range of genetic diseases, but not only that: research is underway for cardiovascular diseases, cancer and autoimmune diseases.
