Published on
Updated
Reading 3 mins.
In the United States, a biological therapy that delays the onset of type 1 diabetes has received approval from the Food and Drug Administration for the first time. A treatment that could significantly improve the lives of young patients detected.
This is a first, and excellent news for people predisposed to type 1 diabetes (about 10% of people with diabetes) that is being played out in the United States: a first treatment designed to delay the onset of type 1 diabetes, comes to be approved by the FDA, the Food and Drug Administration.
The goal ? Delay the attack of insulin-producing cells
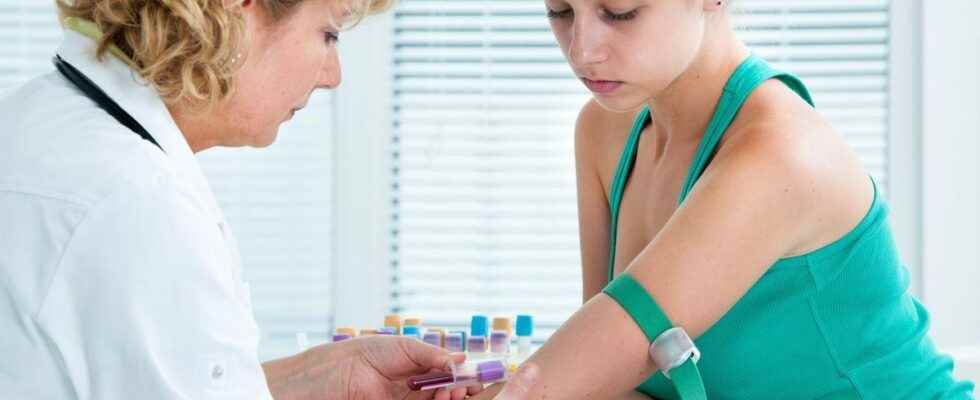
The monoclonal antibody teplizumab, which will be marketed under the brand name Tzield, (ProventionBio and Sanofi) is an intravenous infusion therapy that is believed to target the body’s misdirected attack on its own insulin-producing cells.
With type 1 diabetes, a person’s immune system attacks cells called beta cells in the pancreas which produce insulin, a hormone that helps blood sugar enter cells, where it is used as a source of energy. Without insulin, blood sugar ends up building up in the bloodstream and breaking down body fat and muscle. According to the labs, Tzield would therefore hold back the disease before symptoms appear by stopping the autoimmune disease process and the underlying destruction of beta cells.
The idea of preventive treatment is therefore based on the protection of cells, to buy time before the people concerned become dependent on insulin.
Two weeks of treatment to delay diabetes for years
The treatment is presented in a single course of 14 days of infusions which each last from 30 to 60 minutes.
In its clinical trials, administration of Tzield delayed participants’ progression to diabetes by just over two years. But the benefits lasted much longer for some. The study includes the case of Mikayla Olsten, a young girl who was screened for diabetes after her 9-year-old sister, Mia, was diagnosed with diabetes. Mikayla was not sick, but she had four of the five types of autoantibodies that doctors look for to assess a person’s risk.
The 15-year-old girl when she joined the study and received teplizumab is now 21 and her condition has not progressed for six years.
Screening, the other lever to activate to delay type 1 diabetes
According to a scientific statement from JDRF, the Endocrine Society and the American Diabetes Association, when a person has markers of autoimmune disease and episodes of uncontrolled blood sugar, the five-year risk of progression to symptomatic disease insulin-dependent is 75%. The lifetime risk of developing insulin-dependent diabetes is almost 100%.
“Testing is becoming a very big issue, because what we know is that about 85% of type 1 diagnoses today are in families who have no known family history,” said Aaron J. Kowalski, head of JDRF.“Our goal is to screen the general population with blood tests to look for markers of the disease”.
With Tzield, doctors would therefore screen individual family members of people with type 1 diabetes to see if they have these specific antibodies. If antibody levels are high and it looks like the person is on the verge of developing diabetes, treatment will delay this process.
According to INSERM figures, type 1 diabetes represents about 10% of diabetes cases in France and worldwide. Half of the cases occur before the age of 20. Currently in France, the incidence of type 1 diabetes is around 15 cases per 100,000 children under the age of 15.
For Olivier Bogillot, head of general medicine at Sanofi in the United States, the approved treatment is therefore essential: “When you have the ability, with treatment, to simply delay the onset of disease, you can change how the quality of life is affected for families and for these children.”