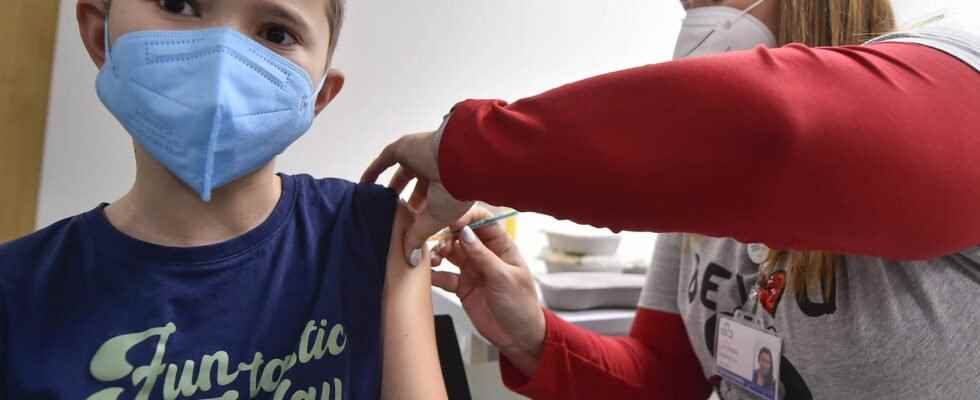
CHILDREN AND TEENS VACCINATION. All children aged 5 to 11 can now access anti-Covid vaccination in France. The health authorities have set rules and insist on the solidity of the scientific opinions rendered recently.
All children aged 5 to 11 can now be vaccinated in France against the coronavirus. The government officially announced it on Wednesday, December 22, through Olivier Véran. The Minister of Health wanted to be cautious and asked for several opinions from scientific bodies to ensure that the vaccination of children was of a general benefit while being safe: that of the National Consultative Ethics Committee (CCNE), that’s why I wrote to you of the High Authority for Health, then the Orientation Council for the vaccine strategy (COSV). Appointments have been opened.
For all the scientists consulted, the benefit / risk ratio of childhood vaccination turns out to be “favorable” on an individual level, even in healthy children. The HAS also considers that the vaccination of children “could potentially reduce the impact of subsequent waves by reducing the circulation of the virus in the general population”, and points out that it is useful to prioritize the vaccination of schoolchildren aged under. 12 years.
No, the vaccination of children under 12 is only carried out on a voluntary basis and with the explicit agreement, via form and signature, of one of the parents. A parent should also be present when the vaccine dose is administered. It is also possible to request a serological test to find out if the child has already contracted Covid-19, given the fact that the asymptomatic forms are numerous in 5-11 year olds.
There is no question for the moment of establishing a health pass for 5-11 year olds. The opinion of the National Consultative Ethics Committee (CCNE), dated December 16, insists that “this vaccination of children must be a proposal and not an obligation, and must not be included in a health pass”.
Based on safety data from the Phase 3 Pfizer vaccine study cited by the US Food and Drug Administration (FDA) and the Vaccine Strategy Guidance Council (COSV) on December 6, the vaccine against the coronavirus is “safe and well tolerated” by children. On a sample of 2268 children aged 5 to 11 years (included in double blind, 2 vaccinated for 1 placebo), “the most frequent side effects consisted of pain and an inflammatory reaction at the point of sting, headaches, chills or fever “but” no complications have been reported, including no case of myocarditis “, one could read. The document specified, however, that the size of this study was “too small to be able to detect adverse and severe effects whose frequency would be of the order of 1/1000 or less” and considered “necessary to collect more data on possible side effects based on real-life data from countries that have already started immunizing children aged 5-11 years. ” Data that has since started to deliver results.
L’opinion of the National Consultative Ethics Committee (CCNE), published for its part in mid-December, indeed indicated that “the safety data” in real life “, in other words statistics on the undesirable effects actually observed after the injection of the vaccine in the countries having already started the vaccination, are “reassuring after a complete vaccination schedule”. All this while asking, in France, “a pharmaco-epidemiological follow-up in the age group of 5 to 11 years” by the services of the State with “a vision of medium-long term “.
L’opinion of the Haute Autorité de Santé (HAS) published on December 20 came to detail a significant number of these data on the adverse effects of the vaccine on children. Recalling the analysis of clinical safety data, it also looked at international pharmacovigilance data relating to more than 7 million vaccinations in children under 12 years of age in the United States (more than 2 million two-dose complete vaccines) and about 107,500 carried out in Israel. Two countries which began immunizing children in November. Globally, there are more than 10 million children aged 0 to 14 around the world who make it possible to display conclusions “still limited but reassuring”, indicates the HAS. Of these millions of children vaccinated, it reports a total of “3233 adverse events” according to North American databases, “of which the majority of them (97%) were not serious”. For the remaining 3%, there are 2 deaths occurring “in children with a heavy medical history” and “14 cases of myocarditis”, one of the most feared side effects.
“About thirty adverse events, all serious”, have also been reported from all over the world to the European database (Eudravigilance) following “a vaccination with the Pfizer vaccine between November 16 and December 12, 2021”, specifies the HAS which lists “3 neurological effects, 2 myocarditis, 1 anaphylactic reaction, 1 respiratory arrest and 1 death”. Regarding this death which occurred in the United States, “the evaluation of the association between BNT162b2 (the Pfizer vaccine – Editor’s note) and the circumstances leading to the reported fatal outcome is impossible”, specifies the document.
While the Covid in children is particularly rapid during this 5th wave and the fear of seeing them transmit the virus to adults and the elderly is strong, the government has decided to vaccinate 5-11 year olds. one of the new axes of health policy in the coming weeks. If “children are much less affected than adults by severe forms of Covid-19, they are not exempt”, thus indicated on December 6 the Council of orientation of the vaccine strategy. From Jean Castex to Olivier Véran, the government also insists that the 6 million children aged 5 to 11 must now be vaccinated, considering “that the benefit-risk balance is favorable”. “I am a living example. It was my 11-year-old daughter who gave me the virus a few weeks ago,” Jean Castex argued on France Bleu on December 12. However, the government has opted for the incentive and not for the obligation in the face of parents who are sometimes reluctant.
The World Health Organization (WHO) itself observes, according to a communication as of December 7, that the age groups between 5 and 14 are currently the most affected by the pandemic, with rates sometimes two to three times higher than in the rest of the population. “The health risks are not limited to the children themselves. As the school holidays approach, we also need to recognize that children infect their parents and grandparents at home, with a 10 times higher risk for these adults from developing serious illness, being hospitalized or dying when not vaccinated, “said Dr Hans Henri P. Kluge, WHO director for Europe, in the statement, which added : “Vaccination of young children not only reduces their role in the transmission of Covid-19, but also protects them from pediatric severity, whether associated with long Covid or multisystem inflammatory syndromes”.
L’opinion of the National Consultative Ethics Committee (CCNE) dated December 16, considers the vaccination of children aged 5 to 11 “ethically acceptable”, as long as “the implementation of this vaccination policy” is done “in respect of the informed choice of parents and without constraint “, that this campaign is not” organized in haste, but prepared with health professionals and parents of students, in order to respect the specific needs of the child “and that” the organization of vaccination in children does not interfere with the booster dose in adults, which remains essential and priority “.
Many parents against immunizing children
According to a Elabe poll for BFMTV, L’Express and SFR unveiled Thursday, December 16 and more generally concerning the end of year celebrations, 7 parents out of 10 would be against the vaccination of their own children. To the question “Are you in favor or against vaccination against Covid-19 of children aged 5 to 11?”, The parents of children in this age group answer 68% that they are against it ( 47% “very opposed” and 21% “somewhat opposed”), while 24% of parents say they are “somewhat favorable” and 7% “very favorable”. A paradox when we know that out of a larger sample, 51% of all respondents said they were in favor of vaccinating the youngest (survey carried out on a sample of 1,000 people representative of the French population aged 18 and over. ).
Investigation CoviPrev, the last wave of which was also published on December 16 (read here), gives slightly higher results on the vaccination of children but with a balance still slightly negative: the parents of children aged 5 to 11 are 43% to indicate their intention to have them vaccinated, a rate which increases according to CSP (53% in CSP +), age (51% in parents over 40), or vaccination status (49% in parents already vaccinated). This survey has been carried out in successive waves by Public Health France since the first confinement, with a view to monitoring changes in behavior and mental health during the Covid-19 epidemic.
In Europe, Denmark and Austria have already taken the plunge in childhood vaccination. In Germany, where opponents of vaccination remain mobilized, several regions, including the capital Berlin and Bavaria, launched Wednesday, December 15. Hungary has also launched its campaign for 5-11 year olds, like Greece or Spain, one of the good pupils of vaccination in Europe. Other European countries, such as Italy, Poland, the Baltic countries or even Slovakia, the Czech Republic, Portugal and Switzerland will start their campaign in the coming days, AFP lists.
Outside of Europe, Canada and the United States and even Israel have also authorized the vaccination of children very early this fall. China, Chile, Argentina, Venezuela and Colombia vaccinate children from 3 years old, Cuba and Nicaragua from 2 years old.
Children are vaccinated in France with a third of a dose of Pfizer vaccine, usually reserved for adults (10 micrograms, against 30 for adults). “The bottles, which will contain ten doses, will have an orange cap to differentiate them from adult doses (with a purple cap) and to avoid any error”, specifies The world December 15.
Pfizer was the first vaccine to be approved by the authorities. The US Food and Drug Administration (FDA) Review Panel found in mid-October that Pfizer-BioNTech vaccines presented more benefits than risks for this age group. Pfizer presented the results of a clinical trial to the FDA that showed the vaccine to be 90.7% effective in preventing symptomatic forms of the disease in children aged 5 to 11. Results reported in France in a opinion of the Vaccine Strategy Orientation Council December 6. The Moderna vaccine, for its part, is still in phase III of its clinical trial, but the first results are promising and an authorization request has been made.