Published on
Updated
Reading 6 min.
in collaboration with
Ivan Pourmir (medical oncologist)
Lung, breast, prostate cancer… French oncologists are calling for an end to the classification of cancers by organ. In the age of precision medicine, this classification would no longer make sense and would result in a loss of opportunity for millions of patients. A point of view which remains the subject of debate according to Dr Ivan Pourmir, oncologist and member of the Doctissimo expert committee.
In an article published in the prestigious journal Nature, physician-researchers from Gustave Roussy and the University of Paris-Saclay believe that it is urgent to move towards a biological classification of metastatic cancers. According to them, current segmentation sometimes prevents access to innovative treatments for millions of patients around the world.
A molecular classification rather than by organ
Cancer of the lung, liver, breast, stomach… the classification by organ is found in the teaching of doctors, their specialty, the organization of care, the conduct of clinical studies, marketing authorizations the drug market. If it appears logical a priori, it would be meaningless today because very many cancers share identical molecular mechanisms, independently of the organ where they appear.
The idea is not new, we already mentioned it in 2015, but this time it is supported by renowned oncologists in one of the most prestigious reference journals. “The authoritative classification in oncology, based on the organ in which the cancer appeared, no longer corresponds to the therapeutic advances made in recent years. Worse, it is now sometimes a barrier that prevents certain patients from accessing appropriate treatment.“, explains Professor Fabrice Barlesi, general director of Gustave Roussy.
Precision medicine, through the advent of targeted therapies and immunotherapy in particular, has in fact made molecular profiling of the tumor and its biological and immune characterization essential. It is from this data that the treatments are determined. Numerous studies have highlighted common characteristics shared by several types of organ cancers. The authors thus highlight encouraging results in preliminary clinical trials of conjugated antibodies to treat patients who over-express the HER2 protein, regardless of the organ affected.
Loss of opportunity for millions of patients
According to them, this (r)evolution would constitute an emergency for the sole benefit of patients. “So many arguments which support the necessary reclassification of metastatic cancers, that is to say those spread at a distance, elsewhere than in the organ of origin, and which account for approximately 60 to 90% of cancer deaths.” argue the doctors.
Because these doctors are convinced, the current classification has an impact for millions of patients, in particular by preventing them from accessing innovative treatment. Today, the health authorities of different countries give marketing authorizations organ by organ, which leads to segmentation of clinical trials. According to them, this condition can make it difficult and delay obtaining results (by requiring the recruitment of thousands of patients who have both the mutation sought in the same organ to have sufficient statistical power) and a new classification could rationalize and limit the number of clinical trials. They take the example of olaparib, an anti-parp compound authorized for ovarian cancer in 2014, for breast cancer in 2018, for pancreatic and prostate cancer in 2020, etc.
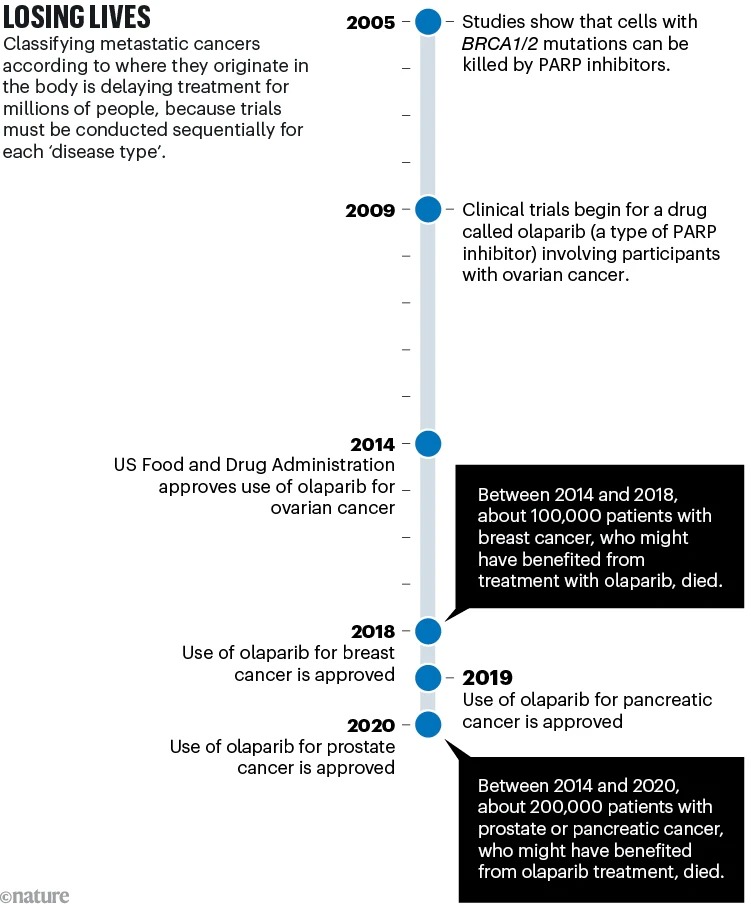
Things are already moving in this direction: in 2017, the American authorities, the FDA, gave marketing authorization for pembrolizumab (Keytruda) on the basis of a specific genetic profile of the tumor, and not of the affected organ.
A simplistic presentation of an already old debate
The editorial thus calls, thanks to this new classification, to reorganize research, the organization of clinical trials including cancers characterized solely by their biological characteristics, the management of patients via trans-organ teams and the teaching of oncology. An evolution that will be facilitated by the advent of artificial intelligence and the provision of effective and affordable molecular profiling methods for all tumors. The argument and reasoning are so logical that one might be surprised that this development has not already been implemented. Well, because there is also room for debate, this development has divided experts for many years.
Historically, the classification of cancers by organ was the result of the tools made available (microscope, coloring, etc.) which made it possible to reveal biological differences which are not necessarily limited to the sole search for mutations with treatments. existing. The way a tumor develops in breast tissue is not necessarily the same as in lung tissue. Taking into account the microenvironment of tumors (primary or metastases) remains an important element. “Research is also moving in this direction of taking into account the environment of the tumor and the characteristics of the patient, in particular their immune characteristics.” specifies Dr. Ivan Pourmir, oncologist at the Georges-Pompidou European Hospital. According to this expert, member of the Doctissimo expert committee, freeing oneself from the organ of origin of the tumor must be considered on a case-by-case basis. The question is whether certain molecular alterations will profoundly reshape the biology of the cancers concerned, to such an extent that they transcend the characteristics of the organ of origin and make it possible to consider a common treatment. For example, this could be the case for microsatellite instability but it is much more questionable for HER2 alterations.
Distinguish therapeutic interests from economic issues
On the other hand, the generalization of so-called “basketball” clinical trials which would bring together cancers of multiple organs would pose several problems, according to our expert. “Determining that a targeted therapy has response rates in many cancers – that is, a preliminary impact on the growth of a tumor – is one thing, saying that this therapy will have an effect on survival is another thing and to say that it provides a benefit compared to the standard of care is another” underlines our expert who highlights the importance of focusing on the design of the clinical study before it is even launched so that it can attest to the effectiveness of the therapy in the long term, that this effectiveness is validated in each organ of origin and in relation to the best treatment currently available. Numerous examples remind us that we must remain humble in relation to our current ability to predict the benefit of a drug on the basis of a theoretical mechanism : clinical trials show that certain treatments do not work even though they had everything to succeed on paper, and conversely “targeted” therapies are effective in cancers where their target is not detected. Finally, mixing all types of cancers in a trial which only aims to show that “it works” does not necessarily make it possible to advance treatment. If we take a simplistic metaphor: to go 10 km, a scooter can “walk” but on the one hand , it will not necessarily be the best solution for everyone and on the other hand, not the most efficient compared to other means of transport.
The benefit of manufacturers in conducting simpler clinical trials making it possible to reach a larger “market” with extremely expensive therapies is obvious, that of patients sometimes remains to be proven. These very expensive therapies represent a considerable market mainly in Europe and especially in the United States which can “afford” these treatments. The benefits for the latter are also 2 to 3 times greater in the United States than in Europe. And this is where health authorities have an essential role according to our expert: “Ideally, the European Medicines Agency but especially the Food and Drug Administration in the United States should be able to validate in advance the way in which the study will be conducted and tell the manufacturer upstream if this or that criterion is not met. respected or does not allow the evaluation of effectiveness compared to the reference treatment, then you will not have marketing authorization for your medicine with us” recommends our expert.
As we can see, the establishment of a new classification of cancers remains the subject of therapeutic, economic and intellectual debates which do not allow us to arrive at a single Manichean option: by organ or by molecular reality. More than an opposition, the solution would certainly be to better reconcile these two approaches.
