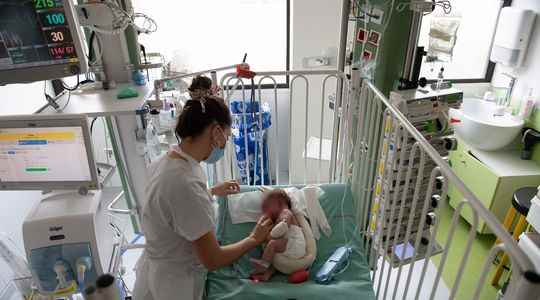
It is a disease of unprecedented magnitude for several years that currently affects France and other European countries. The bronchiolitis epidemic has further intensified in recent days with us, with hospitalizations higher than the previous three seasons and equivalent to the pre-Covid peak, health authorities observed on Thursday, November 3.
Currently, there is no vaccine for this common and highly contagious respiratory disease in infants. But treatments are being developed. The European Union (EU) has approved a preventive treatment for bronchiolitis, nirsevimab, announced on Friday the groups AstraZeneca and Sanofi, which are developing this drug. The European Commission has approved this treatment “for the prevention of respiratory syncytial virus (RSV) infections in infants”, detailed the French group Sanofi in a press release. RSV is one of the viruses that causes bronchiolitis, a disease that mainly affects babies and which, although generally mild, causes symptoms that are often spectacular and sometimes require hospitalization.
Nirsevimab, sold under the name “Beyfortus”, is not strictly speaking a vaccine, but works with the same preventive intention: administered in a single injection, it aims to prevent the occurrence of bronchiolitis. This is a treatment with synthetic antibodies, which directly provides the body with the weapons to fight against the disease and which makes it possible to confer so-called passive immunity on the infant. In contrast, a vaccine allows the body to develop these antibodies itself.
The approval of this treatment, which obtained a favorable opinion last September from the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency, marks an important first in the fight against bronchiolitis. However, it will not be distributed quickly enough to influence this season’s epidemic. “Beyfortus will be available for the next season – 2023 – of bronchiolitis,” Sanofi told AFP.
“RSV prevention is within reach”
It is the first drug to be able to prevent severe forms of bronchiolitis in all babies. Another preventive treatment, also produced by the Swedish-British group AstraZeneca, already exists, but it is only indicated for at-risk or premature children. Other treatments of this type should follow.
Around 30 vaccines or monoclonal antibodies are currently in clinical trials, according to a summary published this summer by the Lancet Infectious Diseases. The British GSK and the Americans Moderna and Johnson & Johnson, in particular, are working on vaccines against RSV. “RSV prevention is within reach”, summed up the authors of the document published by the Lancet Infectious Diseasesrecalling that this virus is the second cause of infant mortality in the world, mainly in poor or middle-income countries.
The American group Pfizer announced on Tuesday positive results for newborns and infants from a clinical trial on a vaccine against RSV, responsible for bronchiolitis, administered to the mother during pregnancy. According to the results of this phase 3 test revealed by the company, the vaccine was found to be approximately 82% effective in preventing serious cases in a baby’s first three months, and approximately 69% within six months. following.
The trial, however, did not conclude that the vaccine reduced non-severe cases in a “statistically significant” way, even if the tests show some clinical effectiveness, the laboratory indicates. Based on these results, which have not been reviewed by independent scientists, Pfizer plans to seek authorization for the vaccine in pregnant women by the end of the year in the United States and then in other countries.