Published on
Updated
Reading 1 min.
To ensure that the use of Ozempic is reserved for people with type 2 diabetes, the ANSM has implemented enhanced monitoring of the drug. Explanations.
On Wednesday, the National Agency for the Safety of Medicines and Medicare announced “enhanced surveillance” of this star drug from TikTok, which is the subject of supply tensions.
Supply tensions on the Ozempic
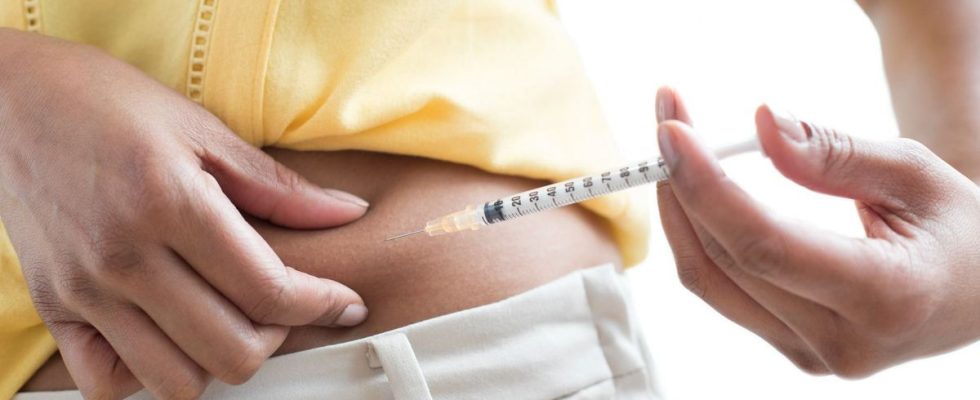
For several months, Ozempic – a drug for patients with type 2 diabetes – has been touted on social media, including TikTok, as an effective “slimming solution”. Hundreds of videos – showing women injecting Ozempic – are circulating on the network.
The problem ? Patients affected by diabetes may find it difficult to find this medicine.
“The diversion of this medicine for weight loss has a direct impact on its availability for diabetic patients and may cause, or exacerbate, supply tensions depriving them of this essential treatment.“, recalls the National Agency for the Safety of Medicines (ANSM).
In this context, the ANSM and the Health Insurance indicate that Ozempic will be the subject of a “enhanced monitoring” in France, by monitoring:
- sales and reimbursement data from the national health data system (SNDS);
- reports of non-compliant use and reports of adverse effects to regional pharmacovigilance centres.
Potentially serious side effects
According to data from the national system, from October 1, 2021 to September 30, 2022, 215,000 patients received and used the drug Ozempic. Among them, 2185 were not diabetic.
Faced with the misuse of the molecule, the ANSM recalls that its use is not without danger:
“Ozempic can cause potentially serious side effects, such as gastrointestinal upset, pancreatitis or hypoglycaemia“.