A new method is being developed to detect cancer cells in the blood. The new method, which is aimed to be used instead of needle biopsy, which is a painful and costly method for the patient, has come to an end. Thanks to the ‘smart chip’ technology developed by METU and which has been working for 7 years, cancer cells circulating in the blood can be detected with a simple blood test.
“NEW TECHNOLOGY IS THE FIRST IN MANY AREAS”
METU Department of Electrical and Electronics Engineering Faculty Member and General Manager of Micro Biosystems Prof. Dr. Haluk Külah stated that Mikro Biyosistemler was founded in 2015 at METU Technopolis.
Külah explained that they continue their international clinical research activities through the company called “Cellsway”, which they established last year in Alderley Park, a technopark that hosts important biotechnology companies in England.

Stating that cancer cells that leave the tumor mass and pass into the blood are called “circulating tumor cells (CTC),” Prof. Dr. Külah said that a very high-precision system is required to separate these cells from the blood, and that the new technology they have developed includes many firsts in this field.
“NEEDLE BIOPSY IS PAINFUL AND COSTLY FOR THE PATIENT”
Pointing out that the method, which is routinely used in the diagnosis and follow-up of cancer, and a surgical procedure in needle biopsy, a sample of the patient’s cancerous tissue is taken, is a painful and costly method for the patient, Külah said, He said they had captured his cells.
“CALLS CANCER CELLS ON ONE SIDE AND NON-CANCER CELLS ON ONE SIDE”
Stating that they can reach information about the metastasis and recurrence risks of cancer and the treatment process, Haluk Külah gave the following information:
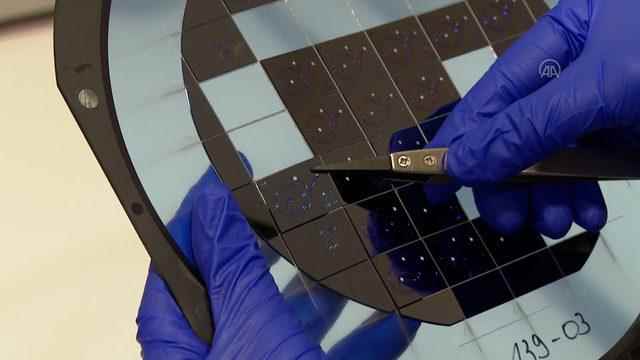
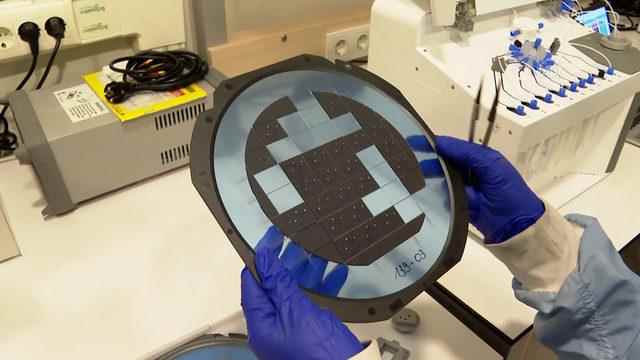
“The design and patent of the silicon-based chips that we developed with MEMS technology to catch cancer cells in blood analysis belong entirely to us. We call these chips smart chips. There are small micro channels like capillaries inside the smart chips. We pass blood cells one by one through these channels, and meanwhile, we pass the blood cells inside the chip. “The mechanical structures separate the cancer cells on one side and the non-cancer cells on the other side. We also collect information about the type of cancer and which type of chemotherapy will work by performing some analyzes on the sample containing the actual cancer cells.”

“WE HAVE COME TO THE STAGE WHERE WE CAN DO CLINICAL STUDY”
prof. Dr. Haluk Külah stated that the system they developed has high accuracy rates and outperforms its competitors.
Concerning the stage they have reached in the technology they have been working on for seven years, Külah said, “Our device and research activities have been completed. We have reached the stage where we can perform clinical studies in laboratories. We have recently started clinical studies on 300 patients. Thus, we will be able to follow up the diagnosis and treatment of cancer with blood tests on real patients for research purposes. ” said.
“PATIENTS CAN HAVE THESE TESTS WITH THEIR EXISTING HEALTH INSURANCE”
Scientific Director of the project, Biochemistry Specialist Assoc. Dr. Ebru Özgür stated that the system they have been working on since 2015 has reached the stage where it can be tested in laboratories after the validation processes, including preliminary clinical studies, have been completed.
Stating that they will start working with patient samples at the clinical stage, Özgür said, “We will set up our system in laboratories and hospitals in the near future.” said.
Stating that they can obtain information about the tumor directly from the blood sample in their method called “liquid biopsy” they have developed, Özgür said, “We provide information to the doctor about whether this treatment works and which treatment may be appropriate in a patient who is started chemotherapy, radiotherapy or targeted drug therapy with the analysis of cancer cells. With these results, they can guide the patient.” he said.
Ebru Özgür stated that the system they developed was used for “research” purposes and that they installed the system in some centers in Ankara and Istanbul, and gave the following information:
“Our study will include breast cancer, colon cancer and non-small cell lung cancer patients. We will also take samples from healthy individuals. We will present their results in a comparative way. Our study will be completed within a year, and then the validation of the process will be completed. The device we developed, As it falls under the category of in vitro diagnostic device, we will make our applications for CE and FDA approval. Once we get our approvals, patients will be able to have these tests done with their existing health insurance. By the end of this year, cancer patients who are treated in certain hospitals, provided that they have the approval of the doctors, will be able to receive blood tests within the scope of our clinical study. will be included in the scope of follow-up with the analysis.
Source: AA