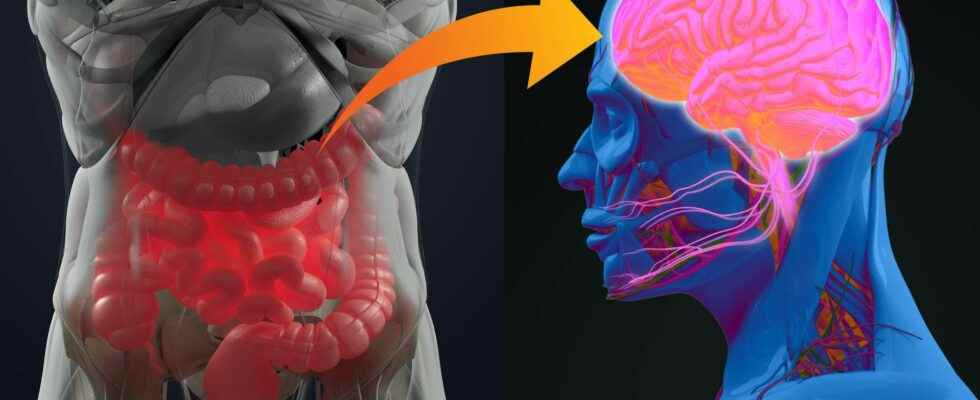
the microbiota intestinal is the largest reservoir of bacteria of the organism. More and more studies show how much the host and its intestinal microbiota are dependent on each other, and underline the importance of the gut-brain axis. At the Institut Pasteur, neurobiologists from the Perception and Memory Unit (Institut Pasteur/CNRS), immunobiologists from the Microenvironment and immunity (Institut Pasteur/Inserm), and microbiologists from the Biology and genetic of the bacterial wall (Institut Pasteur/CNRS/Inserm) pooled their expertise to understand how gut bacteria may have a direct effect on the activity of certain neurons of brain.
Scientists were particularly interested in the NOD2 receptor (Nucleotide Oligomerization Domain) which is present inside the cells, in particular immune cells. This receptor detects the presence of muropeptides, compounds of the bacterial walls, which can be considered as products derived from the intestinal microbiota. Moreover, it was already known that variants of embarrassed encoding the NOD2 receptor are associated with certain diseases of the digestive system, such as Crohn’s diseasebut also to some neurological diseases Where mood disorders.
When the NOD2 receiver receiver is faulty
These data did not yet make it possible to conclude on a direct relationship between the functioning of neurons in the brain and the bacterial activity of the intestine. That’s what put in light the consortium of scientists in this new study, published in Science last April 15.
Using brain imaging techniques, the scientists first observed, in mice, that the NOD2 receptor is expressed by neurons in different regions of the brain, and in particular in a center called thehypothalamus. They then discovered that these same neurons see their electrical activity repressed when they encounter bacterial muropeptides from the intestine. Muropeptides are released by bacteria as they proliferate.
” Muropeptides present in the intestine, blood and brain are considered the markers of bacterial proliferation explains Ivo G. Boneca, Head of the Biology and Genetics of the Bacterial Wall Unit at the Institut Pasteur (CNRS/Inserm).
Conversely, in the case where the NOD2 receptor is defective, these neurons are no longer repressed by the muropeptides; the brain then loses control of food intake and body temperature. As a result, the mice gain weight and are more likely to develop a diabetes type 2, especially in older females.
A new therapeutic approach against metabolic or neurological disorders
Surprisingly, the scientists showed here that it is the neurons that directly perceive the bacterial muropeptides, whereas this task is generally assigned to the cells of the immune system. ” It is amazing to discover that bacterial fragments act directly on such a strategic nerve center as the hypothalamus, known to manage vital functions such as body temperature, reproduction, hunger, or thirst. commented Pierre-Marie Lledo, CNRS researcher and head of the Perception and Memory Unit at the Institut Pasteur.
Thus, neurons seem to detect bacterial activity (proliferation and death) to directly measure the impact of food intake on theecosystem intestinal. ” It is possible that excessive food intake or a particular food promotes the exaggerated expansion of certain bacteria or pathogensand thus endangers the intestinal balance”emphasizes Gérard Eberl, head of the Microenvironment and Immunity Unit at the Institut Pasteur (Inserm).
Given the impact of muropeptides on the neurons of the hypothalamus and the metabolism, one can wonder about their role in other brain functions, and thus understand the association between certain brain diseases and genetic variants of NOD2. This discovery paves the way for new interdisciplinary projects for the three research teams and ultimately for new therapeutic approaches against diseases of the brain, or metabolic diseases such as diabetes and theobesity.
A link between the cells of our brain and our intestines
The stem cells are essential for the regeneration of damaged, diseased or aging cells. And researchers are now showing the existence of a hormone common to the maintenance of optimal functioning of different stem cells, present in the intestines and the brain.
Article of Nathalie Mayerpublished on April 18, 2019
A few months ago, a study reported a neural connection between our brain and our intestines. Today, researchers from therutgers university (United States) seem to want to make a new link between these two organs essential to our health: stem cells. Or rather a hormone essential for maintaining stem cells both in the brain and in the intestines.
Remember that a proliferation of stem cells in the intestine can lead to the appearance of a colorectal cancer. At the level of the brain, the phenomenon can cause anxiety disorders or cognitive impairment. And researchers are now highlighting the importance of one and the same growth factor-2 analogous toinsulin (IGF-II).
Same factor for different stem cells
“The discovery that there is a common factor in several populations of adult stem cells is remarkable”, comments Steven Levison. This would therefore be essential for cognitive function, sense of smell and renewal of the mucous membrane of thesmall intestine in adults.
To reach these conclusions, the researchers deleted the gene responsible for the production of IGF-II in mice. A rapid deletion of this gene led to the death of the mice within a week, after dramatic weight losses. Slower deletion of the gene allowed the mice to survive – other stem cells in the gut having taken over – but with learning and memory deficits, increased anxiety and loss of mind.smellhalf of the neural stem cells having been lost in two areas of the brain.
Your gut is directly connected to your brain
Researchers have demonstrated the existence of a neural connection between the intestine and the brainstem, located just below the brain. Until now, only a slower hormonal communication had been described to explain the control of hunger.
Article of Marie-Celine Ray published on 09/24/2018
Our brain receives information from our five senses: vision, hearing, touch, taste and smell. These messages are quickly transported thanks to sensory fibers which transmit electrical messages: this is how, as soon as you open the door from your house, you can see the mess in your living room or immediately smell a good smell of baking chocolate cake.
L’intestine must also send messages to the brain to tell it whether it is full or not, whether to eat or not. For this, it uses hormonal messages, that is to say molecules released into the blood. At the level ofepithelium intestinal, there are cells called “enteroendocrine” which serve as sensors sensory, and “feel” the nutrients present. Nutrients in the gut stimulate the release of hormones, to inform the brain, but minutes or even longer after theingestion of food.
Until now it was thought that these sensory cells acted only through the slow pathway of hormone as the cholecystokinin. The researchers suspected the existence of a faster mechanism because the sensory cells present in the intestine have similarities with those of the tongue and nose. For example, these cells emit an electrical signal if they are stimulated.
In this new research published in Science, researchers at Duke University wanted to better understand the circuitry that connects the gut and the brain. For this they used a virus of the rage marked with a green fluorescence that they injected into thestomach mice in order to follow its path from the gut to the brain. The rabies virus is known to infect neurons.
Intestinal cells have synapses with the vagal system
The researchers observed that the virus passed through the vagus nerve to arrive at brainstem and there was only one synapse between the gut and the brainstem! Additionally, the researchers showed that enteroendocrine cells have presynaptic proteins. In vitrothey cultured these intestinal sensory cells with mouse vagus nerve neurons: the neurons connected to the intestinal cells. The researchers gave sugar to these cells to create a stimulus: a message was transmitted from the enteroendocrine cells to the vagal neurons thanks to the connections created!
Diego Bohórquez, one of the authors of this work, declared in a communicated from duke university “Scientists talk about appetite in terms of minutes and hours. We’re talking seconds here. » This has implications for the search for obesity therapies because many of the appetite-suppressing molecules that have been studied target slow-acting hormones, not synapses fast acting. “That’s probably why most of them failed. »
The researchers suspected the glutamatea neurotransmitter involved in taste and smell, to play a role in conveying the message. When they blocked the release of glutamate at the level of the intestinal sensory cells, there was no longer any message. Therefore, enteroendocrine cells do not only play a hormonal role.
Through synapses with the vagus nerve, these sensory cells directly connect the lumen of the intestine to the brainstem, located at the base of the brain. They send quick messages about the presence of nutrients, like sugar, using glutamate as a neurotransmitter.
Interested in what you just read?