Published on
updated on
Reading 3 min.
Prescription medication, not reimbursed, and strictly supervised: the launch in France of the flagship drug against obesity, Wegovy, is not being done lightly. An ally to fight obesity… but under the surveillance of the National Medicines Safety Agency.
The long-awaited Wegovy, an anti-obesity drug, is finally arriving in France. Yes, it constitutes a real ally against this disease, but be careful, not for just any patient!
Wegovy: a powerful ally, but under close surveillance
This launch comes against a backdrop of exploding demand for a new generation of effective treatments for weight loss: GLP-1 analogues (aGLP-1), which includes Wegovy.
This class of drugs mimics an intestinal hormone that stimulates insulin secretion and provides a feeling of fullness.
In a message sent to AFP, the general director of the France subsidiary of Novo Nordisk, Etienne Tichit, “hopes that this arrival will allow many French patients to enter a care pathway including a low-calorie diet and increased physical activity which remains essential“.
But the ANSM indicates in a separate press release “restrict the conditions of prescription and delivery of all aGLP-1 indicated in the treatment of obesity“.
Who can benefit from it?
Therefore, treatment initiation prescriptions must be provided by specialists in endocrinology-diabetology-nutrition. Renewals can, however, be carried out by general practitioners.
The agency also asks doctors to comply with the care pathway of the High Authority for Health, that is to say to “prescribe aGLP-1 indicated in the treatment of obesity to patients with an initial body mass index (BMI) greater than or equal to 35 kg/m2, aged less than 65 years“.
This medication should only be used after failure of nutritional support and in combination with a low-calorie diet and physical activity.
For the medicine policeman, it is a matter of “secure the use of aGLP-1 indicated in the treatment of obesity, in a context of potential misuse of these products“, notably “diversion for aesthetic purposes” by people who do not have weight-related health problems.
Treatment not reimbursed… and not given
Wegovy is, to date, not registered for reimbursement and its price is therefore set freely, as in most of the fifteen countries where it is already marketed.
Based on its other anti-obesity drug marketed in France, Saxenda (liraglutide), which is not currently evaluated for reimbursement, the French subsidiary of Novo Nordisk estimated the price of the Wegovy treatment “between 9 and 12 euros per day.
Up to 10,000 obese patients had early access to Wegovy in France, according to the manufacturer, as part of early access which allows them to benefit from innovative treatments more than a year before their official marketing.
This access authorized between July 2022 and September 2023 allowed them to have free use until the end of October 2024. The laboratory will extend this measure “until the end of January 2025” so as to “not leave the approximately 7,000 without a solution”. patients still under treatment.
But it is very unlikely that on that date, the negotiations on the price with the economic committee for health products (CEPS) will have been successful, since Wegovy must be re-evaluated “by the end of the year” by the HAS taking into account the results of a new study (Select) which showed a reduction in the risk of major adverse cardiovascular events with this treatment.
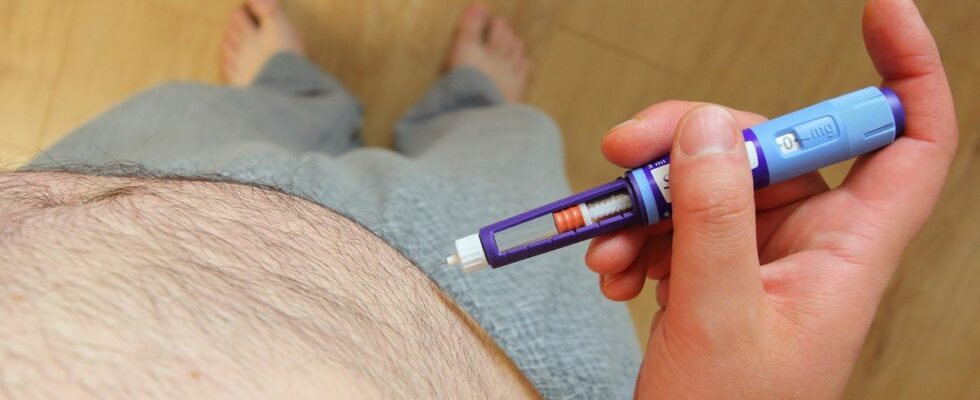
This medication, which consists of a weekly injection with progressive dosage, was authorized by the European Medicines Agency in early 2022 for the management of obesity in adults and since 2023 for adolescents over 12 years old.
NO to diets, YES to WW!
Very encouraging results
The results arouse enthusiasm since the weight loss is, according to the HAS, around 17%, but its use is accompanied by undesirable effects such as nausea, diarrhea or even vomiting.
Its active substance, semaglutide, is the same as that of the antidiabetic Ozempic also manufactured by Novo Nordisk, but in obesity it is used at higher dosages.
These two products made the fortune of the Danish laboratory. The largest European capitalization is investing billions to boost its global production.
Obesity now affects more than a billion people worldwide, according to a study published in March in the British medical journal The Lancet.