Posted ,
Reading 3 mins.
This is a world first! A team of researchers from the University Hospital of Lille is currently conducting an extraordinary clinical trial to treat one of the most common neurodegenerative diseases: Parkinson’s disease. The innovation is linked to the delivery system of the neurotransmitter, dopamine, directly into the brain of patients.
Parkinson’s disease and dopamine, what is the link?
No less than 200,000 French people are affected by Parkinson’s disease, as well as more than 8 million people worldwide. The occurrence of this disease is associated with “the disappearance of dopaminergic neurons, responsible for significant motor disorders”. Some symptoms therefore occur due to a lack of dopamine in the brain. Dopamine is a neurotransmitter: it is a biochemical molecule allowing the cells of the nervous system to communicate with each other.
To rebalance the dopamine level, the current treatment is based on the repeated intake of a drug, L-Dopa, orally. However, as the authors point out, its action has a limited duration and entails “highly disabling motor complications in 50% of patients after 5 years and in 80% of patients after 10 years.” Contrary to popular belief :
- Uncontrolled movements are linked to an overdose of this treatment;
- Underdosing causes more characteristic symptoms of the disease to reappear, such as slowness, stiffness or tremors.
To solve this problem, dopamine would have to be continuously delivered into the brains of patients, similar to the regular release of insulin in diabetic patients.
This is why the DIVE clinical trial was born, to offer a therapeutic alternative and bring comfort to the patient.
An innovative pump to release dopamine
As Professor David DEVOS, from the University Hospital of Lille, from the Neuroscience and Cognition Inserm pole and co-founder of InBrain Pharma, the Lille biotechnology start-up to fight against brain diseases, explains, “dopamine is a fragile molecule, which oxidizes and degrades very quickly in the open air”. It was therefore necessary to take up a major challenge and find a suitable solution in order to preserve the properties of the neurotransmitter.
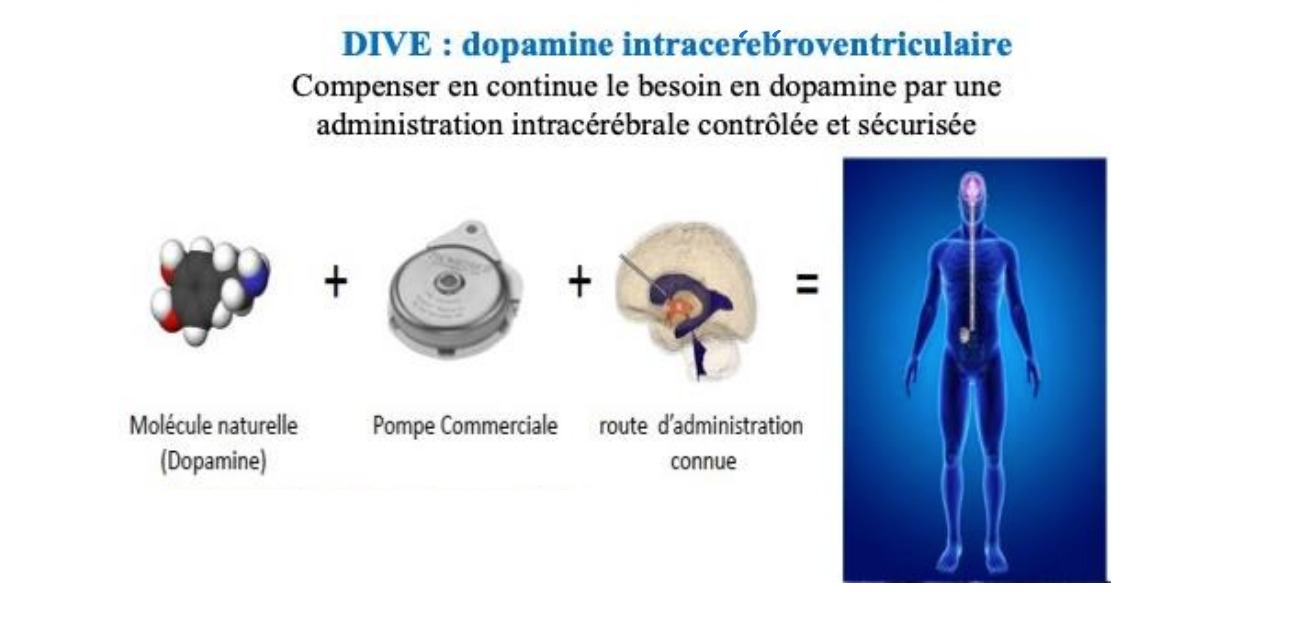
The researchers came up with the idea of “develop its administration in anaerobic conditions (without oxygen to preserve it) and intracerebrally, to patients with Parkinson’s disease at the stage of complications of oral treatment (i.e. after 5 to 10 years of evolution)”.
This test, unique in the world, is currently under development and is based on a new manufacturing process. Indeed, as explained by his collaborator, Professor Caroline MOREAU, dopamine is “stored in a pump implanted under the skin in the abdominal region to which a thin catheter is connected allowing dopamine to be locally distributed in the cerebral ventricles avoiding peripheral adverse effects”.
A “revolutionary” test
The first results are very encouraging, as they confirm a “high safety and a very promising clinical effect”, note the authors. For this clinical trial, the volunteers were divided into two groups: some patients received the DIVE solution while others received the conventional treatment. Each person recruited must write down their observations at home in a diary. “From moderate doses of around 100 mg/24h, a clear reduction in the effects of overdose of more than 50% is observed in favor of a doubling of the ideal control period”. The researchers make another observation:the control of motor fluctuations seems even more important, around 80% of the ideal control period”.
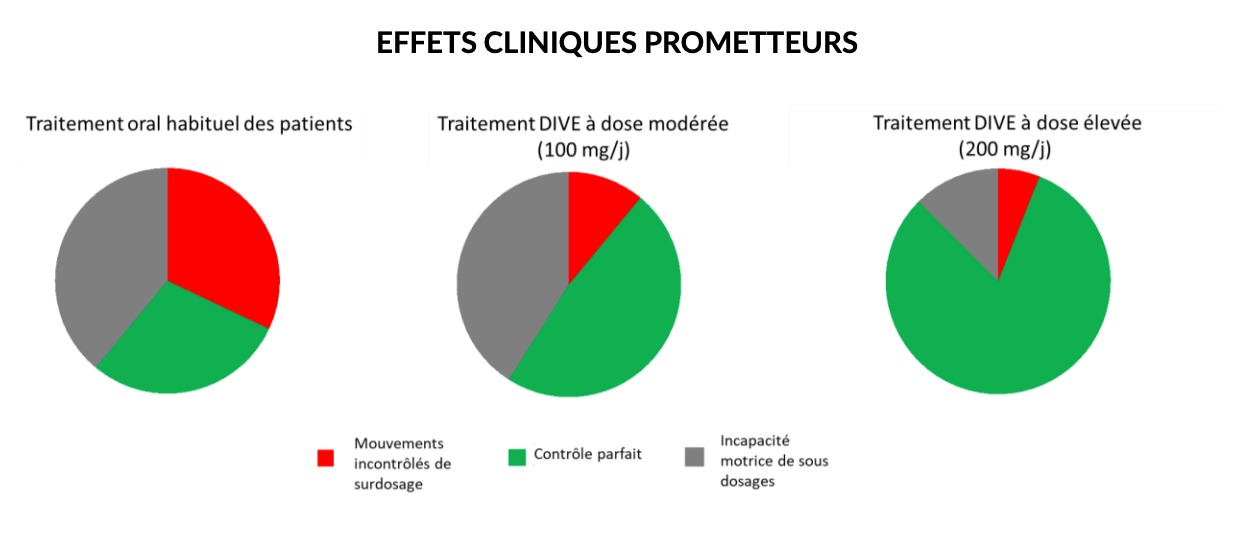
In other words, the uncontrolled movements associated with overdose as well as the motor disability caused by underdose are significantly reduced thanks to this innovative delivery system. Also, the higher the dose of the DIVE treatment, the more significant this reduction.
The other benefit of this innovative therapeutic concept is that the pump is integrated inside the body, providing ease and comfort to the patient. This invisible device should be refilled every 1-2 weeks.
The next step now is to initiate a larger phase III clinical trial, which will likely “obtaining marketing authorization and commercialization of the DIVE treatment”.
