Published on
Updated
Reading 4 min.
A very aggressive brain tumor, glioblastoma is associated with a terrible prognosis. But immunotherapy, which has already revolutionized the treatment of several cancers, could change the situation. In a few days, two teams of researchers announced spectacular results.
Glioblastoma, a formidable cancer
Each year, around 3,500 new cases of glioblastoma are diagnosed in France. Mainly affecting adults, it is more common in men than in women. 70% of cases concern people aged 45 to 70.
Affecting brain cells, glioblastoma is an aggressive cancer, when it is classified as grade 3 or 4. Survival of affected patients is limited and there are currently only a few treatments available to shrink the tumor.
The standard treatment for glioblastoma today consists of surgery when possible and a combination of chemotherapy and radiotherapy as adjuvant treatment. But many ongoing clinical studies aim to evaluate the effectiveness of new therapeutic molecules or new administration combinations. Among them, the use of immunotherapy represents real hope.
Ben Trotman, had 9 months to live. Today he is doing well
In 2022, 41-year-old Ben Trotman is diagnosed with glioblastoma. A first global NEAT clinical trial was quickly included in which immunotherapy is administered before standard treatment of surgery (when possible), radiotherapy and chemotherapy. With current standard treatment, average survival is nine months.
Ben had a very severe reaction to the treatment, a headache so severe that he had to be hospitalized. Dr. Mulholland, who led the trial, thought it was a significant immune response to his tumor. Returning home in early December, he began standard treatment in January and also married his fiancée Emily that month. He continues his monthly chemotherapy.

But last summer, his scan showed no signs of illness. His last scan showed the same thing and today Ben is doing well.
Although the press release University College London Hospitals does not mention the name of the medicine, the page dedicated to the current trial specifies that it is ipilimumab (marketed under the name Yervoy ©). This monoclonal antibody is an inhibitor of the CTLA-4 checkpoint of T lymphocytes, a checkpoint that certain cancers activate to reduce the effectiveness of the lymphocyte. The lymphocytes can then proliferate and infiltrate the tumor to kill the tumor cells. The drug already has indications for certain colorectal, kidney, esophageal, melanoma and mesothelioma cancers.
Today, this unprecedented cure does not, however, provide certainty (the response to immunotherapy depends on each patient), it reinforces the interest of immunotherapy in the face of these formidable tumors.
A spectacular regression with CAR-TEAM cells
A new immunotherapy approach to glioblastoma also makes the front page of the prestigious journal The New England Journal of Medicine. As part of this study, a 72-year-old man diagnosed with glioblastoma benefited from treatment which significantly reduced his tumor in a few days.
In this case, the researchers used CAR-T cells. This highly personalized therapy is based on the use of the patient’s own cells to fight cancer. These cells are extracted, modified to produce proteins on their surface called chimeric antigen receptors, and then injected back into the body to directly target the tumor. Having already proven effective in certain blood cancers, CAR-T cells have limited effectiveness on solid tumors. Because they contain mixed populations of cells, some cancer cells manage to evade detection by the immune system, even after treatment. Faced with this challenge, the team at Mass General Cancer Center combined two previously distinct strategies: CAR-T and bispecific antibodies, known as T cell-engaging antibody molecules (TEAM).
These cells, called CAR-TEAM, were injected into the brains of 3 patients, all already treated with standard radiotherapy and temozolomide chemotherapy and included in the trial after a recurrence of the disease:
- A 74-year-old man saw his tumor regress quickly, but transiently, after a single infusion of new CAR-TEAM cells;
- A 72-year-old man was treated with a single infusion of CAR-TEAM cells. Two days after receiving CAR-TEAM cells, an MRI showed a decrease in tumor size by 18.5%. By day 69, the tumor had shrunk by 60.7% and the response was maintained for more than 6 months;
- A 57-year-old woman was treated with CAR-TEAM cells. An MRI five days after a single infusion of CAR-TEAM cells showed almost complete tumor regression.
Patients tolerated the infusions well (apart from a fever and altered mental status shortly after the infusion).
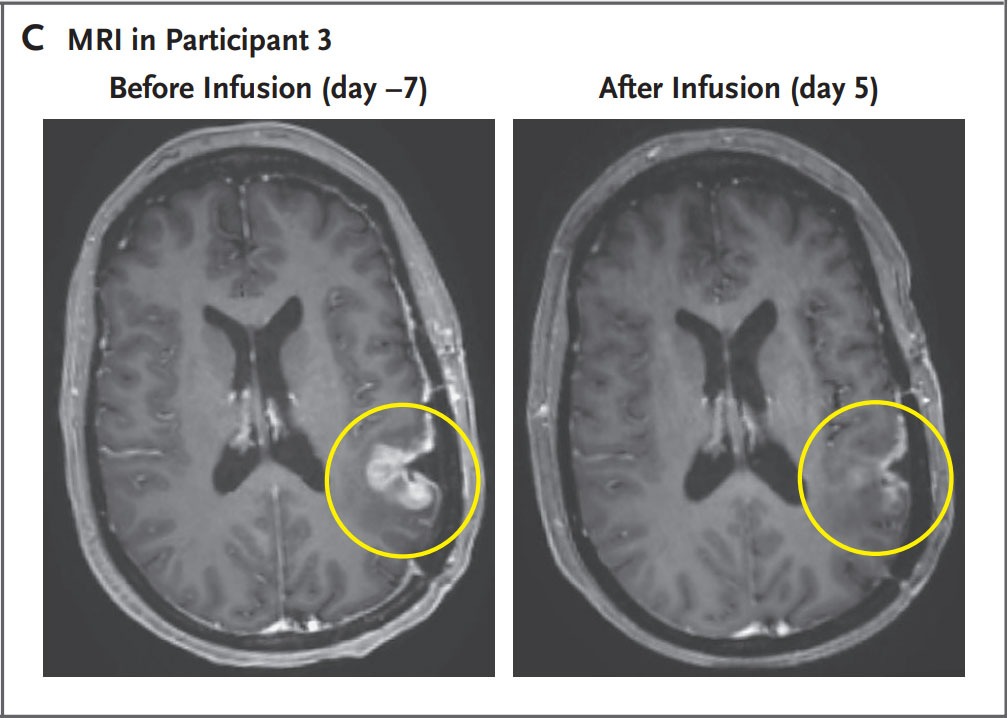
Despite these promising results, the authors eventually observed tumor progression in all cases, although in one case there was no progression for more than six months. They believe this is related to the limited persistence of CAR-TEAM cells in the weeks following infusion. They are working on strategies to prolong the response: serial infusions, preconditioning with chemotherapy, etc.
“We report a spectacular and rapid response in these three patients. Our work to date shows signs that we are making progress, but there is still much to do” said co-author Elizabeth Gerstner, a neuro-oncologist in the Department of Neurology at Massachusetts General Hospital.